IL-10 producing type 2 innate lymphoid cells prolong islet allograft survival
- PMID: 33034128
- PMCID: PMC7645373
- DOI: 10.15252/emmm.202012305
IL-10 producing type 2 innate lymphoid cells prolong islet allograft survival
Abstract
Type 2 innate lymphoid cells (ILC2s) are a subset of ILCs with critical roles in immunoregulation. However, the possible role of ILC2s as immunotherapy against allograft rejection remains unclear. Here, we show that IL-33 significantly prolonged islet allograft survival. IL-33-treated mice had elevated numbers of ILC2s and regulatory T cells (Tregs). Depletion of Tregs partially abolished the protective effect of IL-33 on allograft survival, and additional ILC2 depletion in Treg-depleted DEREG mice completely abolished the protective effects of IL-33, indicating that ILC2s play critical roles in IL-33-mediated islet graft protection. Two subsets of ILC2s were identified in islet allografts of IL-33-treated mice: IL-10 producing ILC2s (ILC210 ) and non-IL-10 producing ILC2s (non-ILC10 ). Intravenous transfer of ILC210 cells, but not non-ILC10 , prolonged islet allograft survival in an IL-10-dependent manner. Locally transferred ILC210 cells led to long-term islet graft survival, suggesting that ILC210 cells are required within the allograft for maximal suppressive effect and graft protection. This study has uncovered a major protective role of ILC210 in islet transplantation which could be potentiated as a therapeutic strategy.
Keywords: IL-10; IL-33; innate lymphoid cells; islet transplantation; type 1 diabetes.
© 2020 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
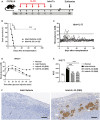
- A
Streptozotocin‐induced diabetic C57BL/6 (H2b) mice were treated with mouse recombinant IL‐33 daily for 5 consecutive days before islet transplantation. On day 0, mice were transplanted with BALB/c (H2d) islets. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected after two consecutive BGLs > 16 mmol/l (mM) after a period of normoglycemia.
- B
Islet graft survival of mice receiving vehicle (PBS) or IL‐33 was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001 vs. islet+vehicle.
- C
Blood glucose level of mice treated with IL‐33 (the horizontal black line indicates a BGL of 16 mmol/l, the threshold for rejection). Each line represents one mouse.
- D
Intraperitoneal glucose tolerance test (IPGTT) was assessed in normal mice, islet transplant mice receiving vehicle (on the day when grafts were considered rejected), and islet transplant mice treated with IL‐33 (at day 30 and day 80 post‐islet transplantation). Data shown are the mean ± SEM (n = 6–9 per group).
- E
Area under the curve (AUC) for IPGTT was assessed. Data shown are the mean ± SEM (n = 6–9 per group), and a one‐way ANOVA was performed, ***P < 0.001.
- F
Representative immunohistochemical staining for insulin in graft samples from mice receiving vehicle or IL‐33. Scale bar = 100 μm.
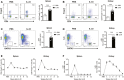
-
C57BL/6 mice were treated with mouse recombinant IL‐33 or PBS daily for 5 consecutive days.
- A, B
Representative FACS analysis showing the proportion of Tregs (CD4+Foxp3+) in the CD4+T‐cell compartment from the spleens (A) and kidneys (B) at day 3 after treatment in C57BL/6 mice receiving PBS (n = 4) or IL‐33 (n = 6).
- C, D
Representative FACS analysis showing the proportion of ILC2s (Lin‐GATA-3+) in the CD45+leukocyte compartment from the spleens (C) and kidneys (D) at day 3 after treatment in C57BL/6 mice receiving PBS (n = 4) or IL‐33 (n = 6).
- E, F
proportion of Tregs (E) and ILC2s (F) in the spleen and kidney in PBS‐injected controls (n = 4) and at weeks 1–12 after IL‐33 treatment (n = 4–6 per IL‐33–treated group).
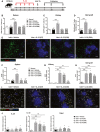
- A
Streptozotocin‐induced diabetic C57BL/6 (H2b) mice with IL‐33 treatment were transplanted with BALB/c (H2d) islets. Mice were sacrificed at day 7, 30, and 80 post‐islet transplantation.
- B–D
Proportion or numbers of CD4+Foxp3+Tregs in the spleens, kidneys, and islet grafts of normal, islet transplant mice receiving vehicle and islet transplant mice with IL‐33 treatment (at day 7, 30, and 80 post‐islet transplantation). Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
- E
Representative confocal microscopy images of immunostaining for CD4, Foxp3, and insulin in islet grafts. Scale bar = 50 μm.
- F–H
Proportion or numbers of ILC2s in the spleens, kidneys, and islet graft of mice with or without IL‐33 treatment. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.
- I
Representative confocal microscopy images of immunostaining for CD127, ST2, CD3, and insulin in islet grafts. Scale bar = 50 μm.
- J
The mRNA expression of IL‐25, IL‐33, and TSLP in islet grafts of mice with or without IL‐33 treatment was examined by qPCR, and expressed relative to the control of each experiment. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.

- A
Streptozotocin‐induced diabetic DEREG C57BL/6 mice were treated with mouse recombinant IL‐33 daily for 5 consecutive days, as well as diphtheria toxin (DT), PC61 or DT+PC61 on days −4 and sletallogr1 prior to and on day 2 post‐islet transplantation. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected.
- B, C
Proportion of CD4+Foxp+Tregs (B) and Lin-GATA‐3+ILC2s (C) from the spleens and kidneys of islet transplant mice receiving vehicle, IL‐33, IL‐33/DT, IL‐33/PC61, or IL‐33/DT/PC61 at day 5 post‐islet transplantation. Data shown are the mean ± SEM (n = 4–5 per group), and a one‐way ANOVA was performed; NS: non‐significant, ***P < 0.001.
- D
Islet graft survival of five groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. *P < 0.05, **P < 0.01.
- E
Data are shown as blood glucose measurement in islet transplant mice treated with IL-33/DT, IL‐33/PC61, or IL‐33/DT/PC61. The horizontal black line indicates a BGL of 16 mmol/l, the threshold for rejection. Each line represents one mouse.

- A, B
ILC210 and non‐IL-10 producing ILC2s were assessed in islet graft (A) and kidney (B) by intracellular IL‐10 staining at day 5 post‐islet transplantation. Data shown are the mean ± SEM (n = 5 per group), and an unpaired t‐test was performed; ***P < 0.001. ST2: suppression of tumorigenicity 2.
- C
IL‐10 reporter C57BL/6 mice were treated with PBS, IL‐33 alone or IL‐33 and IL‐2C daily for 5 consecutive days. Proportion of lin‐CD127+ST2+IL‐10-GFP ILC210 from the kidneys at day 3 after treatment in IL‐10 reporter C57BL/6 mice. Data shown are the mean ± SEM (n = 4–6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
- D
Kidney ILC2s isolated from normal IL‐10 reporter C57BL/6 mice were cultured with medium only or medium with IL‐33 and IL‐2C for 6 days. Proportion of ILC210 was assessed by flow cytometry. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001. ST2: suppression of tumorigenicity 2.
- E
The secreted cytokine IL‐10 was analyzed via ELISA. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001.
- F
Kidney ILC2s isolated from C57BL/6 mice were cultured with medium only or medium with IL‐33 and IL‐2C for 30 min. The expression of phosphorylated STAT5 was examined in kidney ILC2s by flow cytometry. p‐STAT5 (red lines) and isotype controls (gray‐filled areas) are shown. Data represent the mean ± SEM of evaluations of MFI from each group (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001. MFI, mean fluorescence intensity.
- G
The kidney ILC2s were cultured with medium, IL‐33/IL‐2C, or IL-33/IL-2C and STAT5 inhibitor (STAT5‐IN) for 3 days. The secreted cytokine IL‐10 was analyzed via ELISA. Data shown are the mean ± SEM (n = 6 per group), and a one‐way ANOVA was performed; ***P < 0.001.
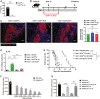
- A
ILC210 and non‐ILC210 were isolated from ex vivo‐expanded kidney ILC2 by flow sorting. ILC210 were transfected with control (ILC210‐C) or IL‐10 CRISPR‐Cas9 (ILC210‐IL-10). IL‐10 was measured in culture supernatant of ILC210‐C and ILC210‐IL-10 via ELISA. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed; ***P < 0.001.
- B
Transfected ILC210‐C and ILC210‐IL-10, and non‐ILC210 were adoptively transferred into diabetic C57BL/6 mice twice at 1 day prior to and 2 days post‐islet transplantation. Mice were sacrificed at the day when grafts were considered rejected.
- C
Transfused ILC2s (CFSE labeled, Green) were observed in islet graft at day 5 post‐islet transplantation. Scale bar = 50 μm. The numbers of CFSE‐labeled ILC2s in the islet graft were counted. Data shown are the mean ± SEM, and a one‐way ANOVA was performed (n = 4 per group). NS, non‐significant.
- D
The mRNA expression of IL‐10 in islet grafts at day 5 post‐islet transplantation was examined by qPCR. Data shown are the mean ± SEM (n = 4 per group), and a one‐way ANOVA was performed; **P < 0.01, ***P < 0.001.
- E
Islet graft survival of four groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001.
- F
CD4+ T cells isolated from C57BL/6 splenocytes were cultured with irradiated splenocytes derived from BALB/c in the presence of ILC210 at the indicated ratios for 4 days. CD4+ T‐cell proliferation was assessed using [3H]‐thymidine incorporation assays. Data shown are the mean ± SEM (n = 4–6 per group).
- G
Neutralizing anti‐IL-10 antibodies or genetic ablation of IL‐10 were used to block the effect of ILC210 on CD4+ T‐cell proliferation. Data shown are the mean ± SEM (n = 6 per group), and an unpaired t‐test was performed. ***P < 0.001.
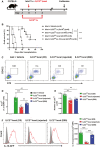
- A
ILC210 were isolated from ex vivo‐expanded kidney ILC2s by flow sorting. ILC210 were co‐transplanted with islet locally or adoptively transferred into diabetic C57BL/6 mice twice at 1 day prior to and 2 days post‐islet transplantation. Mice were sacrificed at day 80 post‐islet transplantation or at the day when grafts were considered rejected.
- B
Islet graft survival of five groups of mice was assessed by monitoring blood glucose and calculated using the Kaplan–Meier method. Cumulative data from two independent experiments are shown. Statistical analysis was performed with a log‐rank test. ***P < 0.001.
- C
To track ILC210 in vivo, CD45.2+ILC210 were co‐transplanted with islets in diabetic CD45.1+C57BL/6 mice. Representative FACS analysis showing the proportion of locally transplanted ILC210 (CD45.2+ST2+) in the total CD45+ cell compartment from islet graft at day 5 and 80 post‐islet transplantation or at the day when grafts were considered rejected.
- D, E
Proportion and numbers of locally transplanted CD45.2+ST2+ILC210 in islet grafts of islet transplant mice over time. Data shown are the mean ± SEM (n = 4–5 per group), and a one‐way ANOVA was performed, **P < 0.01, ***P < 0.001.
- F
The expression of IL‐10-GFP was examined in locally transplanted CD45.2+ST2+ILC210 by flow cytometry. IL‐10-GFP (red lines) and controls (gray‐filled areas) are shown. Data represent the mean ± SEM of evaluations of MFI from each group (n = 4–5 per group), and a one‐way ANOVA was performed; ***P < 0.001. MFI, mean fluorescence intensity.
Similar articles
-
Cell therapy with human interleukin 10-producing ILC2s enhances islet function and inhibits allograft rejection.Am J Transplant. 2025 Sep;25(9):1858-1869. doi: 10.1016/j.ajt.2025.05.023. Epub 2025 May 23. Am J Transplant. 2025. PMID: 40412656
-
Potential correlation of allograft infiltrating group 2 innate lymphoid cells with acute rejection after liver transplantation.Front Immunol. 2022 Jul 28;13:953240. doi: 10.3389/fimmu.2022.953240. eCollection 2022. Front Immunol. 2022. PMID: 35967423 Free PMC article.
-
Group 2 innate lymphoid cells promote TNBC lung metastasis via the IL-13-MDSC axis in a murine tumor model.Int Immunopharmacol. 2021 Oct;99:107924. doi: 10.1016/j.intimp.2021.107924. Epub 2021 Jul 1. Int Immunopharmacol. 2021. PMID: 34217145
-
Regulatory ILC2-Role of IL-10 Producing ILC2 in Asthma.Cells. 2023 Oct 31;12(21):2556. doi: 10.3390/cells12212556. Cells. 2023. PMID: 37947634 Free PMC article. Review.
-
Trained innate lymphoid cells in allergic diseases.Allergol Int. 2021 Apr;70(2):174-180. doi: 10.1016/j.alit.2020.11.007. Epub 2020 Dec 13. Allergol Int. 2021. PMID: 33328130 Review.
Cited by
-
Innate immune cellular therapeutics in transplantation.Front Transplant. 2023;2:1067512. doi: 10.3389/frtra.2023.1067512. Epub 2023 Mar 31. Front Transplant. 2023. PMID: 37994308 Free PMC article.
-
Innate Lymphoid Cells: Role in Immune Regulation and Cancer.Cancers (Basel). 2022 Apr 21;14(9):2071. doi: 10.3390/cancers14092071. Cancers (Basel). 2022. PMID: 35565201 Free PMC article. Review.
-
Navigating challenges in human pluripotent stem cell-derived islet therapy for type 1 diabetes.Front Immunol. 2025 Aug 4;16:1625439. doi: 10.3389/fimmu.2025.1625439. eCollection 2025. Front Immunol. 2025. PMID: 40831568 Free PMC article. Review.
-
IL-10-Producing ILCs: Molecular Mechanisms and Disease Relevance.Front Immunol. 2021 Mar 29;12:650200. doi: 10.3389/fimmu.2021.650200. eCollection 2021. Front Immunol. 2021. PMID: 33859642 Free PMC article. Review.
-
Characterization and Proteomic Analyses of Proinflammatory Cytokines in a Mouse Model of Liver Transplant Rejection.Oxid Med Cell Longev. 2022 Aug 12;2022:5188584. doi: 10.1155/2022/5188584. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35993024 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- U1804167/National Natural Science Foundation of China (NSFC)
- 81570624/National Natural Science Foundation of China (NSFC)
- 81770721/National Natural Science Foundation of China (NSFC)
- 81671752/National Natural Science Foundation of China (NSFC)
- 1141330/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

