Long-term effects of empagliflozin on excitation-contraction-coupling in human induced pluripotent stem cell cardiomyocytes
- PMID: 33034709
- PMCID: PMC7679329
- DOI: 10.1007/s00109-020-01989-6
Long-term effects of empagliflozin on excitation-contraction-coupling in human induced pluripotent stem cell cardiomyocytes
Abstract
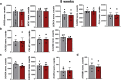
The SGLT2 inhibitor empagliflozin improved cardiovascular outcomes in patients with diabetes. As the cardiac mechanisms remain elusive, we investigated the long-term effects (up to 2 months) of empagliflozin on excitation-contraction (EC)-coupling in human cardiomyocytes derived from induced pluripotent stem cells (iPSC-CM) in a blinded manner. IPSC from 3 donors, differentiated into pure iPSC-CM (4 differentiations), were treated with a clinically relevant concentration of empagliflozin (0.5 μmol/l) or vehicle control. Treatment, data acquisition, and analysis were conducted externally blinded. Epifluorescence microscopy measurements in iPSC-CM showed that empagliflozin has neutral effects on Ca2+ transient amplitude, diastolic Ca2+ levels, Ca2+ transient kinetics, or sarcoplasmic Ca2+ load after 2 weeks or 8 weeks of treatment. Confocal microscopy determining possible effects on proarrhythmogenic diastolic Ca2+ release events showed that in iPSC-CM, Ca2+ spark frequency and leak was not altered after chronic treatment with empagliflozin. Finally, in patch-clamp experiments, empagliflozin did not change action potential duration, amplitude, or resting membrane potential compared with vehicle control after long-term treatment. Next-generation RNA sequencing (NGS) and mapped transcriptome profiles of iPSC-CMs untreated and treated with empagliflozin for 8 weeks showed no differentially expressed EC-coupling genes. In line with NGS data, Western blots indicate that empagliflozin has negligible effects on key EC-coupling proteins. In this blinded study, direct treatment of iPSC-CM with empagliflozin for a clinically relevant duration of 2 months did not influence cardiomyocyte EC-coupling and electrophysiology. Therefore, it is likely that other mechanisms independent of cardiomyocyte EC-coupling are responsible for the beneficial treatment effect of empagliflozin. KEY MESSAGES: This blinded study investigated the clinically relevant long-term effects (up to 2 months) of empagliflozin on cardiomyocyte excitation-contraction (EC)-coupling. Human cardiomyocytes derived from induced pluripotent stem cells (iPSC-CM) were used to study a human model including a high repetition number of experiments. Empagliflozin has neutral effects on cardiomyocyte Ca2+ transients, sarcoplasmic Ca2+ load, and diastolic sarcoplasmic Ca2+ leak. In patch-clamp experiments, empagliflozin did not change the action potential. Next-generation RNA sequencing, mapped transcriptome profiles, and Western blots of iPSC-CM untreated and treated with empagliflozin showed no differentially expressed EC-coupling candidates.
Keywords: EC-coupling; Electrophysiology; Empagliflozin; iPSC-CM.
Conflict of interest statement
SS and LSM receive speaker’s/consultancy honoraria from Boehringer Ingelheim Pharma GmbH. SP, FR, ND, OS, GS, JM, KH, GH, NH, and KSB have nothing to declare.
Figures





References
-
- Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. - DOI - PubMed
-
- McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Belohlavek J, Bohm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukat A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O'Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjostrand M, Langkilde AM, Committees D-HT, Investigators (2019) Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 381:1995–2008 doi: - PubMed
-
- Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B. Empagliflozin reduced mortality and hospitalization for heart failure across the Spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation. 2019;139:1384–1395. doi: 10.1161/CIRCULATIONAHA.118.037778. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

