Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study
- PMID: 33035628
- PMCID: PMC7536538
- DOI: 10.1016/j.jhep.2020.09.024
Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study
Abstract
Background & aims: Chronic liver disease (CLD) and cirrhosis are associated with immune dysregulation, leading to concerns that affected patients may be at risk of adverse outcomes following SARS-CoV-2 infection. We aimed to determine the impact of COVID-19 on patients with pre-existing liver disease, which currently remains ill-defined.
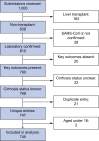
Methods: Between 25th March and 8th July 2020, data on 745 patients with CLD and SARS-CoV-2 (including 386 with and 359 without cirrhosis) were collected by 2 international registries and compared to data on non-CLD patients with SARS-CoV-2 from a UK hospital network.
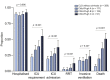
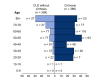
Results: Mortality was 32% in patients with cirrhosis compared to 8% in those without (p <0.001). Mortality in patients with cirrhosis increased according to Child-Pugh class (A [19%], B [35%], C [51%]) and the main cause of death was from respiratory failure (71%). After adjusting for baseline characteristics, factors associated with death in the total CLD cohort were age (odds ratio [OR] 1.02; 1.01-1.04), Child-Pugh A (OR 1.90; 1.03-3.52), B (OR 4.14; 2.4-7.65), or C (OR 9.32; 4.80-18.08) cirrhosis and alcohol-related liver disease (OR 1.79; 1.03-3.13). Compared to patients without CLD (n = 620), propensity-score-matched analysis revealed significant increases in mortality in those with Child-Pugh B (+20.0% [8.8%-31.3%]) and C (+38.1% [27.1%-49.2%]) cirrhosis. Acute hepatic decompensation occurred in 46% of patients with cirrhosis, of whom 21% had no respiratory symptoms. Half of those with hepatic decompensation had acute-on-chronic liver failure.
Conclusions: In the largest such cohort to date, we demonstrate that baseline liver disease stage and alcohol-related liver disease are independent risk factors for death from COVID-19. These data have important implications for the risk stratification of patients with CLD across the globe during the COVID-19 pandemic.
Lay summary: This international registry study demonstrates that patients with cirrhosis are at increased risk of death from COVID-19. Mortality from COVID-19 was particularly high among patients with more advanced cirrhosis and those with alcohol-related liver disease.
Keywords: Acute-on-chronic liver failure; COVID-19; Chronic liver disease; Cirrhosis; SARS-CoV-2.
Copyright © 2020 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


Comment in
-
SARS-CoV-2 in patients with chronic liver disease.Nat Rev Gastroenterol Hepatol. 2020 Dec;17(12):714. doi: 10.1038/s41575-020-00384-3. Nat Rev Gastroenterol Hepatol. 2020. PMID: 33128018 Free PMC article. No abstract available.
-
COVID-19 in Individuals with Chronic Liver Diseases.J Gastrointestin Liver Dis. 2024 Mar 29;33(1):7-10. doi: 10.15403/jgld-5268. J Gastrointestin Liver Dis. 2024. PMID: 38554411
References
-
- Asrani S.K., Devarbhavi H., Eaton J., Kamath P.S. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
-
- Pimpin L., Cortez-Pinto H., Negro F., Corbould E., Lazarus J.V., Webber L. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69:718–735. - PubMed
-
- Albillos A., Lario M., Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385–1396. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

