Evoked potentials reveal neural circuits engaged by human deep brain stimulation
- PMID: 33035726
- PMCID: PMC7722102
- DOI: 10.1016/j.brs.2020.09.028
Evoked potentials reveal neural circuits engaged by human deep brain stimulation
Abstract
Background: Deep brain stimulation (DBS) is an effective therapy for reducing the motor symptoms of Parkinson's disease, but the mechanisms of action of DBS and neural correlates of symptoms remain unknown.
Objective: To use the neural response to DBS to reveal connectivity of neural circuits and interactions between groups of neurons as potential mechanisms for DBS.
Methods: We recorded activity evoked by DBS of the subthalamic nucleus (STN) in humans with Parkinson's disease. In follow up experiments we also simultaneously recorded activity in the contralateral STN or the ipsilateral globus pallidus from both internal (GPi) and external (GPe) segments.
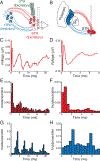
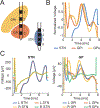
Results: DBS local evoked potentials (DLEPs) were stereotyped across subjects, and a biophysical model of reciprocal connections between the STN and the GPe recreated DLEPs. Simultaneous STN and GP recordings during STN DBS demonstrate that DBS evoked potentials were present throughout the basal ganglia and confirmed that DLEPs arose from the reciprocal connections between the STN and GPe. The shape and amplitude of the DLEPs were dependent on the frequency and duration of DBS and were correlated with resting beta band oscillations. In the frequency domain, DLEPs appeared as a 350 Hz high frequency oscillation (HFO) independent of the frequency of DBS.
Conclusions: DBS evoked potentials suggest that the intrinsic dynamics of the STN and GP are highly interlinked and may provide a promising new biomarker for adaptive DBS.
Keywords: DBS; Globus pallidus; Parkinson’’s disease; Subthalamic nucleus.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest WMG is cofounder, Director and CSO of Deep Brain Innovations LLC. WMG is Director and CSO of NDI Healthcare Fund and receives compensation for these positions. These relationships are reported to the Conflict of Interest Committee at Duke University.
Figures





References
-
- Sutton S, Braren M, Zubin J, John E. Evoked-potential correlates of stimulus uncertainty. Science 1965;150(3700):1187–8. - PubMed
-
- Thorpe S, Fize D, Marlot C. Speed of processing in the human visual system. Nature 1996;381(6582):520–2. - PubMed
-
- Buchsbaum MS, Davis GC, Bunney WE. Naloxone alters pain perception and somatosensory evoked potentials in normal subjects. Nature 1977;270(5638):620–2. - PubMed
-
- Miller CA, Brown CJ, Abbas PJ, Chi S-L. The clinical application of potentials evoked from the peripheral auditory system. Hearing research 2008;242(1):184–97. - PubMed
-
- Parker JL, Karantonis DM, Single PS, Obradovic M, Laird J, Gorman RB, et al. Electrically evoked compound action potentials recorded from the sheep spinal cord. Neuromodulation: Technology at the Neural Interface 2013;16(4):295–303. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

