C. elegans Apical Extracellular Matrices Shape Epithelia
- PMID: 33036165
- PMCID: PMC7712855
- DOI: 10.3390/jdb8040023
C. elegans Apical Extracellular Matrices Shape Epithelia
Abstract
Apical extracellular matrices (aECMs) coat exposed surfaces of epithelia to shape developing tissues and protect them from environmental insults. Despite their widespread importance for human health, aECMs are poorly understood compared to basal and stromal ECMs. The nematode Caenorhabditis elegans contains a variety of distinct aECMs, some of which share many of the same types of components (lipids, lipoproteins, collagens, zona pellucida domain proteins, chondroitin glycosaminoglycans and proteoglycans) with mammalian aECMs. These aECMs include the eggshell, a glycocalyx-like pre-cuticle, both collagenous and chitin-based cuticles, and other understudied aECMs of internal epithelia. C. elegans allows rapid genetic manipulations and live imaging of fluorescently-tagged aECM components, and is therefore providing new insights into aECM structure, trafficking, assembly, and functions in tissue shaping.
Keywords: C. elegans; apical extracellular matrix; cuticle; eggshell; glycocalyx.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
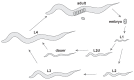
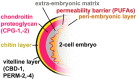
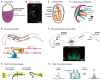


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

