Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy
- PMID: 33036192
- PMCID: PMC7601592
- DOI: 10.3390/cancers12102870
Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy
Abstract
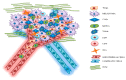
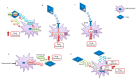
Antitumor therapies have made great strides in recent decades. Chemotherapy, aggressive and unable to discriminate cancer from healthy cells, has given way to personalized treatments that, recognizing and blocking specific molecular targets, have paved the way for targeted and effective therapies. Melanoma was one of the first tumor types to benefit from this new care frontier by introducing specific inhibitors for v-Raf murine sarcoma viral oncogene homolog B (BRAF), mitogen-activated protein kinase (MEK), v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT), and, recently, immunotherapy. However, despite the progress made in the melanoma treatment, primary and/or acquired drug resistance remains an unresolved problem. The molecular dynamics that promote this phenomenon are very complex but several studies have shown that the tumor microenvironment (TME) plays, certainly, a key role. In this review, we will describe the new melanoma treatment approaches and we will analyze the mechanisms by which TME promotes resistance to targeted therapy and immunotherapy.
Keywords: immunotherapy; melanoma; targeted therapy; therapeutic resistance; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

