Dendritic Cell and T Cell Crosstalk in Liver Fibrogenesis and Hepatocarcinogenesis: Implications for Prevention and Therapy of Liver Cancer
- PMID: 33036244
- PMCID: PMC7583774
- DOI: 10.3390/ijms21197378
Dendritic Cell and T Cell Crosstalk in Liver Fibrogenesis and Hepatocarcinogenesis: Implications for Prevention and Therapy of Liver Cancer
Abstract
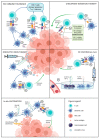
Liver fibrosis is a chronic, highly prevalent disease that may progress to cirrhosis and substantially increases the risk for development of hepatocellular carcinoma (HCC). Fibrotic livers are characterized by an inflammatory microenvironment that is composed of various immunologically active cells, including liver-resident populations (e.g., Kupffer cells, hepatic stellate cells and sinusoidal endothelium) and infiltrating leukocytes (e.g., monocytes, monocyte-derived macrophages, neutrophils and lymphocytes). While inflammatory injury drives both fibrogenesis and carcinogenesis, the tolerogenic microenvironment of the liver conveys immunosuppressive effects that encourage tumor growth. An insufficient crosstalk between dendritic cells (DCs), the professional antigen presenting cells, and T cells, the efficient anti-tumor effector cells, is one of the main mechanisms of HCC tumor tolerance. The meticulous analysis of patient samples and mouse models of fibrosis-HCC provided in-depth insights into molecular mechanisms of immune interactions in liver cancer. The therapeutic modulation of this multifaceted immunological response, e.g., by inhibiting immune checkpoint molecules, in situ vaccination, oncolytic viruses or combinations thereof, is a rapidly evolving field that holds the potential to improve the outcome of patients with HCC. This review aims to highlight the current understanding of DC-T cell interactions in fibrogenesis and hepatocarcinogenesis and to illustrate the potentials and pitfalls of therapeutic clinical translation.
Keywords: HCC; T cells; antigen-presenting cells; checkpoint inhibitors; cirrhosis; dendritic cell vaccine; dendritic cells; fibrosis; immunotherapy; tumor tolerance.
Conflict of interest statement
The lab of F.T. has received research funding from Allergan, Bristol-Myers Squibb, Galapagos, Gilead and Inventiva. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

