Harnessing Mechanosensation in Next Generation Cardiovascular Tissue Engineering
- PMID: 33036467
- PMCID: PMC7599461
- DOI: 10.3390/biom10101419
Harnessing Mechanosensation in Next Generation Cardiovascular Tissue Engineering
Abstract
The ability of the cells to sense mechanical cues is an integral component of "social" cell behavior inside tissues with a complex architecture. Through "mechanosensation" cells are in fact able to decrypt motion, geometries and physical information of surrounding cells and extracellular matrices by activating intracellular pathways converging onto gene expression circuitries controlling cell and tissue homeostasis. Additionally, only recently cell mechanosensation has been integrated systematically as a crucial element in tissue pathophysiology. In the present review, we highlight some of the current efforts to assess the relevance of mechanical sensing into pathology modeling and manufacturing criteria for a next generation of cardiovascular tissue implants.
Keywords: cardiac regeneration; mechanosensing; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
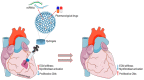
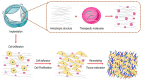

References
-
- Santoro R., Scaini D., Severino L.U., Amadeo F., Ferrari S., Bernava G., Garoffolo G., Agrifoglio M., Casalis L., Pesce M. Activation of human aortic valve interstitial cells by local stiffness involves YAP-dependent transcriptional signaling. Biomaterials. 2018;181:268–279. doi: 10.1016/j.biomaterials.2018.07.033. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

