Non-Invasive Early Molecular Detection of Gastric Cancers
- PMID: 33036473
- PMCID: PMC7600616
- DOI: 10.3390/cancers12102880
Non-Invasive Early Molecular Detection of Gastric Cancers
Abstract
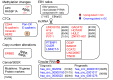
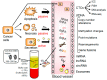
Gastric cancer (GC) is a significant source of global cancer death with a high mortality rate, because the majority of patients with GC are diagnosed at a late stage, with limited therapeutic choices and poor outcomes. Therefore, development of minimally invasive or noninvasive biomarkers which are specific to GC is crucially needed. The latest advancements in the understanding of GC molecular landscapes and molecular biological methods have accelerated attempts to diagnose GC at an early stage. Body fluids, including peripheral blood, saliva, gastric juice/wash, urine, and others, can be a source of biomarkers, offering new methods for the early detection of GC. Liquid biopsy-based methods using circulating sources of cancer nucleic acids could also be considered as alternative strategies. Moreover, investigating gastric juices/washes could represent an alternative for the detection of GC via invasive biopsy. This review summarizes recently reported biomarkers based on DNA methylation, microRNA, long noncoding RNA, circular RNA, or extracellular vesicles (exosomes) for the detection of GC. Although the majority of studies have been conducted to detect these alterations in advanced-stage GC and only a few in population studies or early-stage GC, some biomarkers are potentially valuable for the development of novel approaches for an early noninvasive detection of GC.
Keywords: DNA methylation; circular RNA; extracellular vesicles; gastric juice; gastric wash; liquid biopsy; long noncoding RNA; microRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

