Verbascoside-Rich Abeliophyllum distichum Nakai Leaf Extracts Prevent LPS-Induced Preterm Birth Through Inhibiting the Expression of Proinflammatory Cytokines from Macrophages and the Cell Death of Trophoblasts Induced by TNF-α
- PMID: 33036475
- PMCID: PMC7583932
- DOI: 10.3390/molecules25194579
Verbascoside-Rich Abeliophyllum distichum Nakai Leaf Extracts Prevent LPS-Induced Preterm Birth Through Inhibiting the Expression of Proinflammatory Cytokines from Macrophages and the Cell Death of Trophoblasts Induced by TNF-α
Abstract
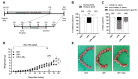
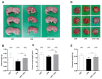
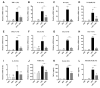
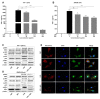
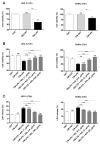
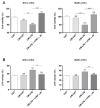
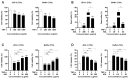
Background: Preterm birth is a known leading cause of neonatal mortality and morbidity. The underlying causes of pregnancy-associated complications are numerous, but infection and inflammation are the essential high-risk factors. However, there are no safe and effective preventive drugs that can be applied to pregnant women. Objective: The objectives of the study were to investigate a natural product, Abeliophyllum distichum leaf (ADL) extract, to examine the possibility of preventing preterm birth caused by inflammation. Methods: We used a mouse preterm birth model by intraperitoneally injecting lipopolysaccharides (LPS). ELISA, Western blot, real-time PCR and immunofluorescence staining analyses were performed to confirm the anti-inflammatory efficacy and related mechanisms of the ADL extracts. Cytotoxicity and cell death were measured using Cell Counting Kit-8 (CCK-8) analysis and flow cytometer. Results: A daily administration of ADL extract significantly reduced preterm birth, fetal loss, and fetal growth restriction after an intraperitoneal injection of LPS in mice. The ADL extract prevented the LPS-induced expression of TNF-α in maternal serum and amniotic fluid and attenuated the LPS-induced upregulation of placental proinflammatory genes, including IL-1β, IL-6, IL-12p40, and TNF-α and the chemokine gene CXCL-1, CCL-2, CCL3, and CCL-4. LPS-treated THP-1 cell-conditioned medium accelerated trophoblast cell death, and TNF-α played an essential role in this effect. The ADL extract reduced LPS-treated THP-1 cell-conditioned medium-induced trophoblast cell death by inhibiting MAPKs and the NF-κB pathway in macrophages. ADL extract prevented exogenous TNF-α-induced increased trophoblast cell death and decreased cell viability. Conclusions: We have demonstrated that the inhibition of LPS-induced inflammation by ADL extract can prevent preterm birth, fetal loss, and fetal growth restriction.
Keywords: Abeliophyllum distichum Nakai; TNF-α; inflammation; macrophage; preterm birth; trophoblast.
Conflict of interest statement
The authors have declared no competing financial interests.
Figures

References
-
- Born Too Soon: The Global Action Report on Preterm Birth. WTO; Geneve, Switzerland: 2012.
-
- Romero R., Roslansky P., Oyarzun E., Wan M., Emamian M., Novitsky T.J., Gould M.J., Hobbins J.C. Labor and infection: II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am. J. Obstet. Gynecol. 1988;158:1044–1049. doi: 10.1016/0002-9378(88)90216-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

