Natural Compound-derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents
- PMID: 33036496
- PMCID: PMC7583968
- DOI: 10.3390/molecules25194582
Natural Compound-derived Cytochrome bc1 Complex Inhibitors as Antifungal Agents
Abstract
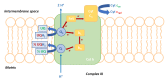
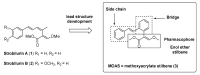
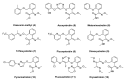
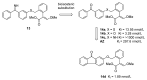
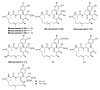
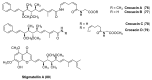
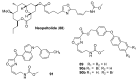
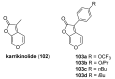
The high incidence of fungal pathogens has become a global issue for crop protection. A promising strategy to control fungal plant infections is based on the use of nature-inspired compounds. The cytochrome bc1 complex is an essential component of the cellular respiratory chain and is one of the most important fungicidal targets. Natural products have played a crucial role in the discovery of cytochrome bc1 inhibitors, as proven by the development of strobilurins, one of the most important classes of crop-protection agents, over the past two decades. In this review, we summarize advances in the exploration of natural product scaffolds for the design and development of new bc1 complex inhibitors. Particular emphasis is given to molecular modeling-based approaches and structure-activity relationship (SAR) studies performed to improve the stability and increase the potency of natural precursors. The collected results highlight the versatility of natural compounds and provide an insight into the potential development of nature-inspired derivatives as antifungal agents.
Keywords: (E)-β-methoxyacrylate; antifungals; cytochrome bc1 complex; natural compounds; strobilurins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

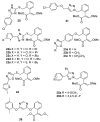
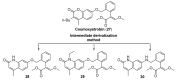
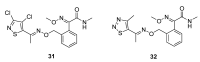
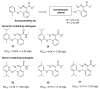



References
-
- Calicioglu O., Flammini A., Bracco S., Bellù L., Sims R. The future challenges of food and agriculture: An integrated analysis of trends and solutions. Sustainability. 2019;11:222. doi: 10.3390/su11010222. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

