Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: a multi-institutional cohort study and meta-analysis
- PMID: 33036617
- PMCID: PMC7547475
- DOI: 10.1186/s12933-020-01148-8
Comparative effectiveness of dulaglutide versus liraglutide in Asian type 2 diabetes patients: a multi-institutional cohort study and meta-analysis
Abstract
Background: Head-to-head comparison of clinical effectiveness between dulaglutide and liraglutide in Asia is limited. This study was aimed to assess the real-world comparative effectiveness of dulaglutide versus liraglutide.
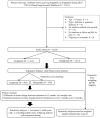
Methods: We conducted a retrospective cohort study by utilizing multi-institutional electronic medical records to identify real-world type 2 diabetes patients treated with dulaglutide or liraglutide during 2016-2018 in Taiwan and followed up until 2019. Effectiveness outcomes were assessed at every 3 months in the 1-year follow-up. Propensity score techniques were applied to enhance between-group comparability. Significant differences in changes of effectiveness outcomes between treatment groups during the follow-up were examined and further analyzed using mixed-model repeated-measures approaches.
Results: A total of 1512 subjects receiving dulaglutide and 1513 subjects receiving liraglutide were identified. At 12 months, significant HbA1c changes from baseline were found in both treatments (dulaglutide: - 1.06%, p < 0.001; liraglutide: - 0.83%, p < 0.001), with a significant between-group difference (- 0.23%, 95% confidence interval - 0.38 to - 0.08%, p < 0.01). Both treatments yielded significant declines in weight, alanine aminotransferase level, and estimated glomerular filtration rate from baseline (dulaglutide: - 1.14 kg, - 3.08 U/L and - 2.08 mL/min/1.73 m2, p < 0.01; liraglutide: - 1.64 kg, - 3.65 U/L and - 2.33 mL/min/1.73 m2, p < 0.001), whereas only dulaglutide yielded a significant systolic blood pressure reduction (- 2.47 mmHg, p < 0.001). Between-group differences in changes of weight, blood pressure, and liver and renal functions at 12 months were not statistically significant.
Conclusions: In real-world T2D patients, dulaglutide versus liraglutide was associated with better glycemic control and comparable effects on changes of weight, blood pressure, and liver and renal functions.
Keywords: Clinical effectiveness and meta-analysis; Dulaglutide; Liraglutide.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Real-world effectiveness of liraglutide versus dulaglutide in Japanese patients with type 2 diabetes: a retrospective study.Sci Rep. 2022 Jan 7;12(1):154. doi: 10.1038/s41598-021-04149-z. Sci Rep. 2022. PMID: 34997102 Free PMC article.
-
Clinical efficacy and predictors of response to dulaglutide in type-2 diabetes.Pharmacol Res. 2020 Sep;159:104996. doi: 10.1016/j.phrs.2020.104996. Epub 2020 Jun 20. Pharmacol Res. 2020. PMID: 32574827
-
Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12-month follow-up in a real-world setting in the United States.Diabetes Obes Metab. 2019 Apr;21(4):920-929. doi: 10.1111/dom.13603. Epub 2019 Jan 9. Diabetes Obes Metab. 2019. PMID: 30520248 Free PMC article.
-
Comparative efficacy and tolerability of currently approved incretin mimetics: A systematic analysis of placebo-controlled clinical trials.Diabetes Obes Metab. 2025 Jul;27(7):3736-3746. doi: 10.1111/dom.16398. Epub 2025 Apr 11. Diabetes Obes Metab. 2025. PMID: 40212008 Free PMC article.
-
Dulaglutide in the treatment of adult type 2 diabetes: a perspective for primary care providers.Postgrad Med. 2016 Nov;128(8):810-821. doi: 10.1080/00325481.2016.1218260. Epub 2016 Aug 12. Postgrad Med. 2016. PMID: 27488824 Review.
Cited by
-
Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes.J Clin Med. 2021 Mar 2;10(5):985. doi: 10.3390/jcm10050985. J Clin Med. 2021. PMID: 33801192 Free PMC article.
-
Real-world evidence on the utilization, clinical and comparative effectiveness, and adverse effects of newer GLP-1RA-based weight-loss therapies.Diabetes Obes Metab. 2025 Apr;27 Suppl 2(Suppl 2):66-88. doi: 10.1111/dom.16364. Epub 2025 Apr 8. Diabetes Obes Metab. 2025. PMID: 40196933 Free PMC article. Review.
-
Comparing the effectiveness of long-term use of daily and weekly glucagon-like peptide-1 receptor agonists treatments in patients with nonalcoholic fatty liver disease and type 2 diabetes mellitus: a network meta-analysis.Front Endocrinol (Lausanne). 2023 Jun 5;14:1170881. doi: 10.3389/fendo.2023.1170881. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37342259 Free PMC article.
-
Efficacy and safety of dulaglutide compared with the first-line hypoglycemic drugs in Asian patients with type 2 diabetes: a systematic review and meta-analysis.Sci Rep. 2022 Oct 31;12(1):18281. doi: 10.1038/s41598-022-22263-4. Sci Rep. 2022. PMID: 36316432 Free PMC article.
-
Once-weekly glucagon-like peptide-1 receptor agonists vs dipeptidyl peptidase-4 inhibitors: cardiovascular effects in people with diabetes and cardiovascular disease.Cardiovasc Diabetol. 2023 Nov 20;22(1):319. doi: 10.1186/s12933-023-02051-8. Cardiovasc Diabetol. 2023. PMID: 37985992 Free PMC article.
References
-
- Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. - DOI - PubMed
-
- Dungan KM, Povedano ST, Forst T, González JGG, Atisso C, Sealls W, Fahrbach JL. Once-weekly dulaglutide versus once-daily liraglutide in metformin-treated patients with type 2 diabetes (AWARD-6): a randomised, open-label, phase 3, non-inferiority trial. Lancet. 2014;384(9951):1349–1357. doi: 10.1016/S0140-6736(14)60976-4. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

