The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy
- PMID: 33036982
- PMCID: PMC10764101
- DOI: 10.1126/sciadv.aat5979
The circadian dynamics of the hippocampal transcriptome and proteome is altered in experimental temporal lobe epilepsy
Abstract
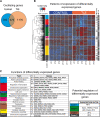
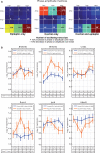
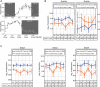
Gene and protein expressions display circadian oscillations, which can be disrupted in diseases in most body organs. Whether these oscillations occur in the healthy hippocampus and whether they are altered in epilepsy are not known. We identified more than 1200 daily oscillating transcripts in the hippocampus of control mice and 1600 in experimental epilepsy, with only one-fourth oscillating in both conditions. Comparison of gene oscillations in control and epilepsy predicted time-dependent alterations in energy metabolism, which were verified experimentally. Although aerobic glycolysis remained constant from morning to afternoon in controls, it increased in epilepsy. In contrast, oxidative phosphorylation increased in control and decreased in epilepsy. Thus, the control hippocampus shows circadian molecular remapping, which is altered in epilepsy. We suggest that the hippocampus operates in a different functioning mode in epilepsy. These alterations need to be considered when studying epilepsy mechanisms, designing drug treatments, and timing their delivery.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Comment in
-
Ticktock-What Is the Seizure Driving Clock?Epilepsy Curr. 2021 Jan 29;21(2):122-123. doi: 10.1177/1535759721989672. eCollection 2021 Mar-Apr. Epilepsy Curr. 2021. PMID: 34025290 Free PMC article. No abstract available.
References
-
- Bass J., Lazar M. A., Circadian time signatures of fitness and disease. Science 354, 994–999 (2016). - PubMed
-
- Brüning F., Noya S. B., Bange T., Koutsouli S., Rudolph J. D., Tyagarajan S. K., Cox J., Mann M., Brown S. A., Robles M. S., Sleep-wake cycles drive daily dynamics of synaptic phosphorylation. Science 366, eaav3617 (2019). - PubMed

