Multiple Transporters and Glycoside Hydrolases Are Involved in Arabinoxylan-Derived Oligosaccharide Utilization in Bifidobacterium pseudocatenulatum
- PMID: 33036985
- PMCID: PMC7688211
- DOI: 10.1128/AEM.01782-20
Multiple Transporters and Glycoside Hydrolases Are Involved in Arabinoxylan-Derived Oligosaccharide Utilization in Bifidobacterium pseudocatenulatum
Abstract
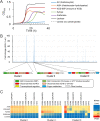
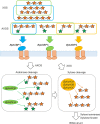
Arabinoxylan hydrolysates (AXH) are the hydrolyzed products of the major components of the dietary fiber arabinoxylan. AXH include diverse oligosaccharides varying in xylose polymerization and side residue modifications with arabinose at the O-2 and/or O-3 position of the xylose unit. Previous studies have reported that AXH exhibit prebiotic properties on gut bifidobacteria; moreover, several adult-associated bifidobacterial species (e.g., Bifidobacterium adolescentis and Bifidobacterium longum subsp. longum) are known to utilize AXH. In this study, we tried to elucidate the molecular mechanisms of AXH utilization by Bifidobacterium pseudocatenulatum, which is a common bifidobacterial species found in adult feces. We performed transcriptomic analysis of B. pseudocatenulatum YIT 4072T, which identified three upregulated gene clusters during AXH utilization. The gene clusters encoded three sets of ATP-binding cassette (ABC) transporters and five enzymes belonging to glycoside hydrolase family 43 (GH43). By characterizing the recombinant proteins, we found that three solute-binding proteins of ABC transporters showed either broad or narrow specificity, two arabinofuranosidases hydrolyzed either single- or double-decorated arabinoxylooligosaccharides, and three xylosidases exhibited functionally identical activity. These data collectively suggest that the transporters and glycoside hydrolases, encoded in the three gene clusters, work together to utilize AXH of different sizes and with different side residue modifications. Thus, our study sheds light on the overall picture of how these proteins collaborate for the utilization of AXH in B. pseudocatenulatum and may explain the predominance of this symbiont species in the adult human gut.IMPORTANCE Bifidobacteria commonly reside in the human intestine and possess abundant genes involved in carbohydrate utilization. Arabinoxylan hydrolysates (AXH) are hydrolyzed products of arabinoxylan, one of the most abundant dietary fibers, and they include xylooligosaccharides and those decorated with arabinofuranosyl residues. The molecular mechanism by which B. pseudocatenulatum, a common bifidobacterial species found in adult feces, utilizes structurally and compositionally variable AXH has yet to be extensively investigated. In this study, we identified three gene clusters (encoding five GH43 enzymes and three solute-binding proteins of ABC transporters) that were upregulated in B. pseudocatenulatum YIT 4072T during AXH utilization. By investigating their substrate specificities, we revealed how these proteins are involved in the uptake and degradation of AXH. These molecular insights may provide a better understanding of how resident bifidobacteria colonize the colon.
Keywords: ABC transporters; GH43; arabinoxylan; bifidobacteria; dietary fiber; glycoside hydrolase; oligosaccharides.
Copyright © 2020 Saito et al.
Figures


References
-
- Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, Price NP, Richardson PM, Mills DA. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc Natl Acad Sci U S A 105:18964–18969. doi: 10.1073/pnas.0809584105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

