SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation
- PMID: 33037188
- PMCID: PMC7545816
- DOI: 10.1038/s41392-020-00334-0
SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation
Abstract
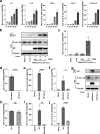
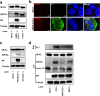
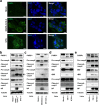
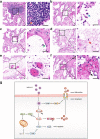
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can lead to respiratory illness and multi-organ failure in critically ill patients. Although the virus-induced lung damage and inflammatory cytokine storm are believed to be directly associated with coronavirus disease 2019 (COVID-19) clinical manifestations, the underlying mechanisms of virus-triggered inflammatory responses are currently unknown. Here we report that SARS-CoV-2 infection activates caspase-8 to trigger cell apoptosis and inflammatory cytokine processing in the lung epithelial cells. The processed inflammatory cytokines are released through the virus-induced necroptosis pathway. Virus-induced apoptosis, necroptosis, and inflammation activation were also observed in the lung sections of SARS-CoV-2-infected HFH4-hACE2 transgenic mouse model, a valid model for studying SARS-CoV-2 pathogenesis. Furthermore, analysis of the postmortem lung sections of fatal COVID-19 patients revealed not only apoptosis and necroptosis but also massive inflammatory cell infiltration, necrotic cell debris, and pulmonary interstitial fibrosis, typical of immune pathogenesis in the lung. The SARS-CoV-2 infection triggered a dual mode of cell death pathways and caspase-8-dependent inflammatory responses may lead to the lung damage in the COVID-19 patients. These discoveries might assist the development of therapeutic strategies to treat COVID-19.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

