Non-motor symptom burden in patients with Parkinson's disease with impulse control disorders and compulsive behaviours: results from the COPPADIS cohort
- PMID: 33037247
- PMCID: PMC7547680
- DOI: 10.1038/s41598-020-73756-z
Non-motor symptom burden in patients with Parkinson's disease with impulse control disorders and compulsive behaviours: results from the COPPADIS cohort
Abstract
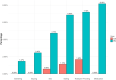
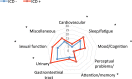
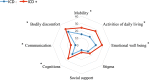
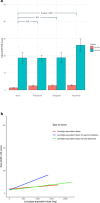
The study was aimed at analysing the frequency of impulse control disorders (ICDs) and compulsive behaviours (CBs) in patients with Parkinson's disease (PD) and in control subjects (CS) as well as the relationship between ICDs/CBs and motor, nonmotor features and dopaminergic treatment in PD patients. Data came from COPPADIS-2015, an observational, descriptive, nationwide (Spain) study. We used the validated Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease-Rating Scale (QUIP-RS) for ICD/CB screening. The association between demographic data and ICDs/CBs was analyzed in both groups. In PD, this relationship was evaluated using clinical features and treatment-related data. As result, 613 PD patients (mean age 62.47 ± 9.09 years, 59.87% men) and 179 CS (mean age 60.84 ± 8.33 years, 47.48% men) were included. ICDs and CBs were more frequent in PD (ICDs 12.7% vs. 1.6%, p < 0.001; CBs 7.18% vs. 1.67%, p = 0.01). PD patients had more frequent previous ICDs history, premorbid impulsive personality and antidepressant treatment (p < 0.05) compared with CS. In PD, patients with ICDs/CBs presented younger age at disease onset, more frequent history of previous ICDs and premorbid personality (p < 0.05), as well as higher comorbidity with nonmotor symptoms, including depression and poor quality of life. Treatment with dopamine agonists increased the risk of ICDs/CBs, being dose dependent (p < 0.05). As conclusions, ICDs and CBs were more frequent in patients with PD than in CS. More nonmotor symptoms were present in patients with PD who had ICDs/CBs compared with those without. Dopamine agonists have a prominent effect on ICDs/CBs, which could be influenced by dose.
Conflict of interest statement
S. Jesús has received honoraria from Abbvie, Bial, Merz, UCB, Italfarmaco and Zambon. She holds the competitive contract "Juan Rodés" supported by the Instituto de Salud Carlos III (JR16/00031). She received grants from the Spanish Ministry of Economy and Competitiveness (PI18/01898) and the Consejería de Salud de la Junta de Andalucía (PI-0459-2018). M. A. Labrador-Espinosa: None. A. D. Adarmes has received honoraria from Abbvie and Italfarmaco. C. Méndez del Barrio has received honoraria from Neuraxpharm. J. C. Martínez-Castrillo has received grants/research support from Allergan, Abbvie, Bial, Ipsen, Italfarmaco, Merz, and Zambon; honoraria or consultation fees from Allergan, AbbVie, Bial, Exeltis, Ipsen, Italfarmaco, Merz, Ipsen, Orion, UCB, and Zambon; and company sponsored speaker’s bureau from Allergan, Abbvie, Bial, Krka, Ipsen, Italfarmaco, Merz, UCB, and Zambon. A. Alonso-Cánovas has received honoraria as lecturer in seminars, simposia and teaching courses, scientific advisory, and travel and congress attendance sponsor with Zambon and Abbvie. P. Sánchez Alonso has received honoraria as lecturer in seminars, symposia and teaching courses, scientific advisory, and travel and congress attendance sponsor with Zambon, Abbvie, Bial. S. Novo-Ponte has received unrestricted educational support from Abbvie, Zambon, Novartis. M. G. Alonso-Losada has received honoraria for educational presentations and advice service by Zambon and Bial. N. López Ariztegui has received honoraria for educational presentations and/or advice service by Abbvie, UCB Pharma, Italfarmaco, Zambon, Bial, and Teva. J. CSegundo Rodríguez: None. M. I. Morales: None. I. Gastón has received research support from Abbvie and Zambon and has served as a consultant for Abbvie, Exelts and Zambon. F. Lacruz Bescos: None. P. Clavero Ibarra: None. J. Kulisevsky has received consulting and lecture fees from Roche, Zambon, Teva and Bial. He got research funding fromRoche, Zambon, Ciberned, Instituto de Salud Carlos III and FundacióLa Maratóde TV3. J. Pagonabarraga has served on advisory or speakers' boards, and received honoraria from UCB, Zambon, AbbVie, Italfarmaco, Allergan, Ipsen and Bial, and received grants from CIBERNED and FIS PI14/02058) (Spanish Government grants) and Fundació La Marató de TV3 20142910. B. Pascual-Sedano has received honoraria by Abbvie and UCB Pharma. P. Martinez-Martin Honoraria: from Editorial Viguera for lecturing in courses; International Parkinson and Movement Disorder Society (IPMDS) for management of the Program on Rating Scales; Air Liquide, Abbvie, and HM Hospitales de Madrid for advice in clinic-epidemiological studies. License fee payments for the King's Parkinson's Disease Pain scale. IPMDS grants for attendance to the IPMDS Congress (2018) and for development and validation of the MDS-NMS. D. Santos-García has received honoraria for educational presentations and/or advice service by Abbvie, UCB Pharma, Lundbeck, KRKA, Zambon, Bial, and Teva. P. Mir has received honoraria from AbbVie, Abbott, Allergan, Bial, Merz, UCB, and Zambon. He has received grants from the Spanish Ministry of Economy and Competitiveness [PI16/01575] co-founded by ISCIII (Subdirección General de Evaluación y Fomento de la Investigación). He also received grants from Fondo Europeo de Desarrollo Regional (FEDER), the Consejería de Economía, Innovación, Ciencia y Empleo de la Junta de Andalucía [CVI-02526, CTS-7685], the Consejería de Salud y Bienestar Social de la Junta de Andalucía [PI-0437-2012, PI-0471-2013], the Sociedad Andaluza de Neurología, the Jacques and Gloria Gossweiler Foundation, the Fundación Alicia Koplowitz, and the Fundación Mutua Madrileña.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

