Engineered cardiac tissues: a novel in vitro model to investigate the pathophysiology of mouse diabetic cardiomyopathy
- PMID: 33037406
- PMCID: PMC8149662
- DOI: 10.1038/s41401-020-00538-8
Engineered cardiac tissues: a novel in vitro model to investigate the pathophysiology of mouse diabetic cardiomyopathy
Abstract
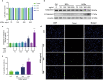
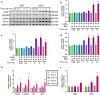
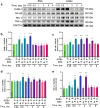
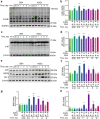
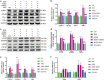
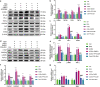
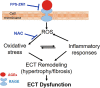
Rodent diabetic models, used to understand the pathophysiology of diabetic cardiomyopathy (DCM), remain several limitations. Engineered cardiac tissues (ECTs) have emerged as robust 3D in vitro models to investigate structure-function relationships as well as cardiac injury and repair. Advanced glycation end-products (AGEs), produced through glycation of proteins or lipids in response to hyperglycemia, are important pathogenic factor for the development of DCM. In the current study, we developed a murine-based ECT model to investigate cardiac injury produced by AGEs. We treated ECTs composed of neonatal murine cardiac cells with AGEs and observed AGE-related functional, cellular, and molecular alterations: (1) AGEs (150 µg/mL) did not cause acute cytotoxicity, which displayed as necrosis detected by medium LDH release or apoptosis detected by cleaved caspase 3 and TUNEL staining, but negatively impacted ECT function on treatment day 9; (2) AGEs treatment significantly increased the markers of fibrosis (TGF-β, α-SMA, Ctgf, Collagen I-α1, Collagen III-α1, and Fn1) and hypertrophy (Nppa and Myh7); (3) AGEs treatment significantly increased ECT oxidative stress markers (3-NT, 4-HNE, HO-1, CAT, and SOD2) and inflammation response markers (PAI-1, TNF-α, NF-κB, and ICAM-1); and (4) AGE-induced pathogenic responses were all attenuated by pre-application of AGE receptor antagonist FPS-ZM1 (20 µM) or the antioxidant glutathione precursor N-acetylcysteine (5 mM). Therefore, AGEs-treated murine ECTs recapitulate the key features of DCM's functional, cellular and molecular pathogenesis, and may serve as a robust in vitro model to investigate cellular structure-function relationships, signaling pathways relevant to DCM and pharmaceutical intervention strategies.
Keywords: FPS-ZM1; N-acetylcysteine; advanced glycation end-products; cardiac fibrosis and hypertrophy; cardiomyopathic in vitro model; diabetic cardiomyopathy; engineered cardiac tissue; inflammation response; oxidative stress.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pr. 2019;157:107843. - PubMed
-
- Rubler SDJ, YuceogluYZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:596–602. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

