Human Hsp90 cochaperones: perspectives on tissue-specific expression and identification of cochaperones with similar in vivo functions
- PMID: 33037995
- PMCID: PMC7736379
- DOI: 10.1007/s12192-020-01167-0
Human Hsp90 cochaperones: perspectives on tissue-specific expression and identification of cochaperones with similar in vivo functions
Abstract
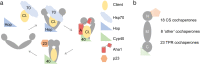
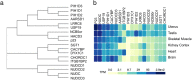
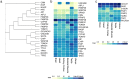
The Hsp90 molecular chaperone is required for the function of hundreds of different cellular proteins. Hsp90 and a cohort of interacting proteins called cochaperones interact with clients in an ATP-dependent cycle. Cochaperone functions include targeting clients to Hsp90, regulating Hsp90 ATPase activity, and/or promoting Hsp90 conformational changes as it progresses through the cycle. Over the last 20 years, the list of cochaperones identified in human cells has grown from the initial six identified in complex with steroid hormone receptors and protein kinases to about fifty different cochaperones found in Hsp90-client complexes. These cochaperones may be placed into three groups based on shared Hsp90 interaction domains. Available evidence indicates that cochaperones vary in client specificity, abundance, and tissue distribution. Many of the cochaperones have critical roles in regulation of cancer and neurodegeneration. A more limited set of cochaperones have cellular functions that may be limited to tissues such as muscle and testis. It is likely that a small set of cochaperones are part of the core Hsp90 machinery required for the folding of a wide range of clients. The presence of more selective cochaperones may allow greater control of Hsp90 activities across different tissues or during development.
Keywords: Aha1; Cdc37; Cochaperone; Hsp90; Molecular chaperone; Tetratricopeptide repeat.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Allosteric regulation of the Hsp90 dynamics and stability by client recruiter cochaperones: protein structure network modeling.PLoS One. 2014 Jan 20;9(1):e86547. doi: 10.1371/journal.pone.0086547. eCollection 2014. PLoS One. 2014. PMID: 24466147 Free PMC article.
-
Activation of the ATPase activity of hsp90 by the stress-regulated cochaperone aha1.Mol Cell. 2002 Dec;10(6):1307-18. doi: 10.1016/s1097-2765(02)00785-2. Mol Cell. 2002. PMID: 12504007
-
Evolution and function of diverse Hsp90 homologs and cochaperone proteins.Biochim Biophys Acta. 2012 Mar;1823(3):607-13. doi: 10.1016/j.bbamcr.2011.09.020. Epub 2011 Oct 8. Biochim Biophys Acta. 2012. PMID: 22008467 Review.
-
Structure, Function, and Regulation of the Hsp90 Machinery.Cold Spring Harb Perspect Biol. 2019 Sep 3;11(9):a034017. doi: 10.1101/cshperspect.a034017. Cold Spring Harb Perspect Biol. 2019. PMID: 30745292 Free PMC article. Review.
-
p23 and Aha1.Subcell Biochem. 2015;78:113-31. doi: 10.1007/978-3-319-11731-7_6. Subcell Biochem. 2015. PMID: 25487019 Review.
Cited by
-
Molecular chaperones: Guardians of tumor suppressor stability and function.Oncotarget. 2024 Oct 1;15:679-696. doi: 10.18632/oncotarget.28653. Oncotarget. 2024. PMID: 39352796 Free PMC article. Review.
-
Saccharomyces cerevisiae as a tool for deciphering Hsp90 molecular chaperone function.Essays Biochem. 2023 Sep 13;67(5):781-795. doi: 10.1042/EBC20220224. Essays Biochem. 2023. PMID: 36912239 Free PMC article.
-
SlHSP17.7 Ameliorates Chilling Stress-Induced Damage by Regulating Phosphatidylglycerol Metabolism and Calcium Signal in Tomato Plants.Plants (Basel). 2022 Jul 18;11(14):1865. doi: 10.3390/plants11141865. Plants (Basel). 2022. PMID: 35890502 Free PMC article.
-
Structural insights of the p97/VCP AAA+ ATPase: How adapter interactions coordinate diverse cellular functionality.J Biol Chem. 2023 Nov;299(11):105182. doi: 10.1016/j.jbc.2023.105182. Epub 2023 Aug 22. J Biol Chem. 2023. PMID: 37611827 Free PMC article. Review.
-
Loss of regulation of protein synthesis and turnover underpins an attenuated stress response in senescent human mesenchymal stem cells.Proc Natl Acad Sci U S A. 2023 Apr 4;120(14):e2210745120. doi: 10.1073/pnas.2210745120. Epub 2023 Mar 29. Proc Natl Acad Sci U S A. 2023. PMID: 36989307 Free PMC article.
References
-
- Alekseev OM, Widgren EE, Richardson RT, O'Rand MG. Association of NASP with HSP90 in mouse spermatogenic cells: stimulation of ATPase activity and transport of linker histones into nuclei. J Biol Chem. 2005;280:2904–2911. - PubMed
-
- Benson DR, Lovell S, Mehzabeen N, Galeva N, Cooper A, Gao P, Battaile KP, Zhu H. Crystal structures of the naturally fused CS and cytochrome b5 reductase (b5R) domains of Ncb5or reveal an expanded CS fold, extensive CS-b5R interactions and productive binding of the NAD(P)(+) nicotinamide ring. Acta Crystallogr D Struct Biol. 2019;75:628–638. - PMC - PubMed
-
- Biebl MM, Riedl M, Buchner J. Hsp90 Co-chaperones form plastic genetic networks adapted to client maturation. Cell Rep. 2020;32:108063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

