A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II
- PMID: 33038326
- PMCID: PMC7854283
- DOI: 10.1016/j.ymthe.2020.09.039
A Phase 2/3 Trial of Pabinafusp Alfa, IDS Fused with Anti-Human Transferrin Receptor Antibody, Targeting Neurodegeneration in MPS-II
Abstract
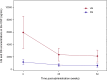
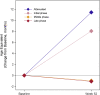
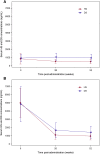
Pabinafusp alfa (JR-141) is a novel enzyme drug that crosses the blood-brain barrier by transcytosis via transferrin receptors. In order to establish its efficacy and safety, a multicenter, single-arm, open-label phase 2/3 clinical trial was conducted in 28 Japanese patients with mucopolysaccharidosis II (MPS-II, Hunter syndrome) by intravenous administrations of 2.0 mg/kg of pabinafusp alfa for 52 weeks. The primary efficacy endpoint was changes in heparan sulfate (HS) concentrations in the cerebrospinal fluid (CSF). Secondary endpoints included assessments of neurocognitive development for central efficacy, and changes in plasma HS and dermatan sulfate (DS) concentrations for peripheral efficacy. HS concentrations in the CSF significantly decreased from baseline to week 52 (p < 0.001), suggesting continuous inhibition of substrate accumulations in the CNS, i.e., hitherto unaddressed progressive neurodegeneration. Evaluations of neurocognitive developments showed positive changes in 21 of the 28 patients. Serum HS and DS concentrations, liver and spleen volumes, and other assessments suggested the peripheral efficacy of pabinafusp alfa was comparable to that of idursulfase. Drug-related adverse events were mild or moderate in severity, transient, and manageable. The results establish delivery across the BBB of pabinafusp alfa as an effective therapeutic for treating both the CNS and peripheral symptoms of patients with MPS-II.
Keywords: Hunter syndrome; anti-human transferrin receptor antibody; blood brain barrier; central nervous system; enzyme-replacement therapy; heparan sulfate; iduronate-2-sulfatase; mucopolysaccharidosis II; neurocognitive development; neurocognitive impairment; neurodegeneration; pabinafusp alfa.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Barichello T., Collodel A., Hasbun R., Morales R. An Overview of Blood-Brain Barrier. In: Barichello T., editor. Neuromethods. Humana Press; 2019. pp. 1–8.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

