Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation
- PMID: 33038331
- PMCID: PMC7725967
- DOI: 10.1016/j.devcel.2020.09.020
Inflammation of the Embryonic Choroid Plexus Barrier following Maternal Immune Activation
Abstract
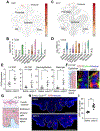
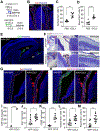
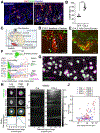
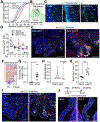
The choroid plexus (ChP) regulates brain development by secreting instructive cues and providing a protective brain barrier. Here, we show that polyI:C-mediated maternal immune activation leads to an inflammatory response in the developing embryonic mouse brain that manifests as pro-inflammatory cerebrospinal fluid (CSF) and accumulation of ChP macrophages. Elevation of CSF-CCL2 was sufficient to drive ChP immune cell recruitment, activation, and proliferation. In addition, ChP macrophages abandoned their regular tiling pattern and relocated to the ChP-free margin where they breached the weakened epithelial barrier. We further found that these immune cells entered from the ChP into the brain via anatomically specialized "hotspots" at the distal tips of ChP villi. In vivo two-photon imaging demonstrated that surveillance behaviors in ChP macrophages had already emerged at this early stage of embryogenesis. Thus, the embryonic ChP forms a functional brain barrier that can mount an inflammatory response to external insults.
Keywords: CCL2; cerebrospinal fluid; choroid plexus; cytokines; immune cells; maternal immune activation; neural development; two-photon imaging.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures

Comment in
-
Maternal Infection Impairs Fetal Brain Development via Choroid Plexus Inflammation.Dev Cell. 2020 Dec 7;55(5):519-521. doi: 10.1016/j.devcel.2020.11.011. Dev Cell. 2020. PMID: 33290691
References
-
- Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL, and Hougaard DM (2012). Amniotic fluid chemokines and autism spectrum disorders: An exploratory study utilizing a Danish Historic Birth Cohort. Brain. Behav. Immun 26, 170–176. - PubMed
-
- Ashwood P, Wills S, and Van de Water J (2006). The immune response in autism: a new frontier for autism research. J. Leukoc. Biol 80, 1–15. - PubMed
-
- Baddeley A, Rubak E, and Turner R (2016). Spatial Point Patterns: Methodology and Applications with R (CRC Press Taylor & Francis Group, LLC; ).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

