Super-Resolution Microscopy and FIB-SEM Imaging Reveal Parental Centriole-Derived, Hybrid Cilium in Mammalian Multiciliated Cells
- PMID: 33038333
- PMCID: PMC7729880
- DOI: 10.1016/j.devcel.2020.09.016
Super-Resolution Microscopy and FIB-SEM Imaging Reveal Parental Centriole-Derived, Hybrid Cilium in Mammalian Multiciliated Cells
Abstract
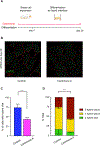
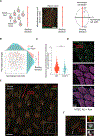
Motile cilia are cellular beating machines that play a critical role in mucociliary clearance, cerebrospinal fluid movement, and fertility. In the airways, hundreds of motile cilia present on the surface of a multiciliated epithelia cell beat coordinately to protect the epithelium from bacteria, viruses, and harmful particulates. During multiciliated cell differentiation, motile cilia are templated from basal bodies, each extending a basal foot-an appendage linking motile cilia together to ensure coordinated beating. Here, we demonstrate that among the many motile cilia of a multiciliated cell, a hybrid cilium with structural features of both primary and motile cilia is harbored. The hybrid cilium is conserved in mammalian multiciliated cells, originates from parental centrioles, and its cellular position is biased and dependent on ciliary beating. Furthermore, we show that the hybrid cilium emerges independently of other motile cilia and functions in regulating basal body alignment.
Keywords: FIB-SEM; airway; appendages; basal bodies; basal foot; centrosome; cilia; electron microscopy; primary ciliary dyskinesia; quantitative imaging; super-resolution imaging.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declarations of Interests The authors declare no competing interests.
Figures





Comment in
-
Basal Feet: Walking to the Discovery of a Novel Hybrid Cilium.Dev Cell. 2020 Oct 26;55(2):115-117. doi: 10.1016/j.devcel.2020.09.018. Dev Cell. 2020. PMID: 33108752
References
-
- Al Jord A, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, and Meunier A (2014). Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516, 104–107. - PubMed
-
- Boisvieux-Ulrich E, Laine MC, and Sandoz D (1985). The orientation of ciliary basal bodies in quail oviduct is related to the ciliary beating cycle commencement. Biol Cell 55, 147–150. - PubMed
-
- Boon M, Wallmeier J, Ma L, Loges NT, Jaspers M, Olbrich H, Dougherty GW, Raidt J, Werner C, Amirav I, et al. (2014). MCIDAS mutations result in a mucociliary clearance disorder with reduced generation of multiple motile cilia. Nature communications 5, 4418. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

