Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study
- PMID: 33038974
- PMCID: PMC7544498
- DOI: 10.1016/S2213-2600(20)30454-9
Clinical impact of molecular point-of-care testing for suspected COVID-19 in hospital (COV-19POC): a prospective, interventional, non-randomised, controlled study
Abstract
Background: The management of the COVID-19 pandemic is hampered by long delays associated with centralised laboratory PCR testing. In hospitals, these delays lead to poor patient flow and nosocomial transmission. Rapid, accurate tests are therefore urgently needed in preparation for the next wave of the pandemic.
Methods: We did a prospective, interventional, non-randomised, controlled study of molecular point-of-care testing in patients aged 18 years or older presenting with suspected COVID-19 to the emergency department or other acute areas of Southampton General Hospital during the first wave of the pandemic in the UK. Nose and throat swab samples taken at admission from patients in the point-of-care testing group were tested with the QIAstat-Dx Respiratory SARS-CoV-2 Panel. Samples taken from patients in a contemporaneous control group were tested by laboratory PCR. The primary outcome was time to results in the full cohort. This study is registered with ISRCTN (ISRCTN14966673) and is completed.
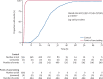
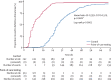
Findings: Between March 20 and April 29, 2020, 517 patients were assessed for eligibility, of whom 499 were recruited to the point-of-care testing group and tested by the QIAstat-Dx Respiratory SARS-CoV-2 Panel. 555 contemporaneously identified patients were included in the control group and tested by laboratory PCR. The two groups were similar with regard to the distribution of sex, age, and ethnicity. 197 (39%) patients in the point-of-care testing group and 155 (28%) in the control group tested positive for COVID-19 (difference 11·5% [95% CI 5·8-17·2], p=0·0001). Median time to results was 1·7 h (IQR 1·6-1·9) in the point-of-care testing group and 21·3 h (16·0-27·9) in the control group (difference 19·6 h [19·0-20·3], p<0·0001). A Cox proportional hazards regression model controlling for age, sex, time of presentation, and severity of illness also showed that time to results was significantly shorter in the point-of-care testing group than in the control group (hazard ratio 4023 [95% CI 545-29 696], p<0·0001).
Interpretation: Point-of-care testing is associated with large reductions in time to results and could lead to improvements in infection control measures and patient flow compared with centralised laboratory PCR testing.
Funding: University Hospitals Southampton NHS Foundation Trust.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Near-patient SARS-CoV-2 molecular platforms: new-old tools for new-old problems.Lancet Respir Med. 2020 Dec;8(12):1161-1163. doi: 10.1016/S2213-2600(20)30451-3. Epub 2020 Oct 8. Lancet Respir Med. 2020. PMID: 33038973 Free PMC article. No abstract available.
-
Implementing rapid diagnostics for COVID-19.Lancet Respir Med. 2021 Jan;9(1):e7. doi: 10.1016/S2213-2600(20)30526-9. Epub 2020 Nov 12. Lancet Respir Med. 2021. PMID: 33189159 Free PMC article. No abstract available.
References
-
- Harding L, Campbell D. Up to 20% of hospital patients with COVID-19 caught it at hospital. May 17, 2020. https://www.theguardian.com/world/2020/may/17/hospital-patients-england-...
-
- BBC Coronavirus: hundreds caught virus in hospitals in Wales. July 22, 2020. https://www.bbc.co.uk/news/uk-wales-53501223
-
- Brendish NJ, Malachira AK, Beard KR, Ewings S, Clark TW. Impact of turnaround time on outcome with point-of-care testing for respiratory viruses: a post hoc analysis from a randomised controlled trial. Eur Respir J. 2018;52 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

