The intracellular renin-angiotensin system: Friend or foe. Some light from the dopaminergic neurons
- PMID: 33039415
- PMCID: PMC7543790
- DOI: 10.1016/j.pneurobio.2020.101919
The intracellular renin-angiotensin system: Friend or foe. Some light from the dopaminergic neurons
Abstract
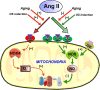
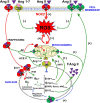
The renin-angiotensin system (RAS) is one of the oldest hormone systems in vertebrate phylogeny. RAS was initially related to regulation of blood pressure and sodium and water homeostasis. However, local or paracrine RAS were later identified in many tissues, including brain, and play a major role in their physiology and pathophysiology. In addition, a major component, ACE2, is the entry receptor for SARS-CoV-2. Overactivation of tissue RAS leads several oxidative stress and inflammatory processes involved in aging-related degenerative changes. In addition, a third level of RAS, the intracellular or intracrine RAS (iRAS), with still unclear functions, has been observed. The possible interaction between the intracellular and extracellular RAS, and particularly the possible deleterious or beneficial effects of the iRAS activation are controversial. The dopaminergic system is particularly interesting to investigate the RAS as important functional interactions between dopamine and RAS have been observed in the brain and several peripheral tissues. Our recent observations in mitochondria and nucleus of dopaminergic neurons may clarify the role of the iRAS. This may be important for the developing of new therapeutic strategies, since the effects on both extracellular and intracellular RAS must be taken into account, and perhaps better understanding of COVID-19 cell mechanisms.
Keywords: ACE/angiotensin receptors/renin angiotensin system; Angiotensin; COVID-19; Cell biology/structural biology; Cell signaling/signal transduction; Intracrine; Mitochondria; Nucleus; Oxidant stress; Oxidative stress.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors report no declarations of interest.
Figures

References
-
- Abadir P.M., Foster D.B., Crow M., Cooke C.A., Rucker J.J., Jain A., Smith B.J., Burks T.N., Cohn R.D., Fedarko N.S., Carey R.M., O’Rourke B., Walston J.D. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14849–14854. - PMC - PubMed
-
- AbdAlla S., Lother H., Abdel-tawab A.M., Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. - PubMed
-
- Albiston A.L., McDowall S.G., Matsacos D., Sim P., Clune E., Mustafa T., Lee J., Mendelsohn F.A., Simpson R.J., Connolly L.M., Chai S.Y. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001;276:48623–48626. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

