Estimating the Lifetime Benefits of Treatments for Heart Failure
- PMID: 33039448
- PMCID: PMC7720789
- DOI: 10.1016/j.jchf.2020.08.004
Estimating the Lifetime Benefits of Treatments for Heart Failure
Abstract
Objectives: This study compared ways of describing treatment effects. The objective was to better explain to clinicians and patients what they might expect from a given treatment, not only in terms of relative and absolute risk reduction, but also in projections of long-term survival.
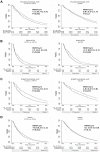
Background: The restricted mean survival time (RMST) can be used to estimate of long-term survival, providing a complementary approach to more conventional metrics (e.g., absolute and relative risk), which may suggest greater benefits of therapy in high-risk patients compared with low-risk patients.
Methods: Relative and absolute risk, as well as the RMST, were calculated in heart failure with reduced ejection fraction (HFrEF) trials.
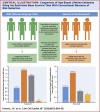
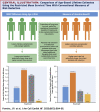
Results: As examples, in the RALES trial (more severe HFrEF), the treatment effect metrics for spironolactone versus placebo on heart failure hospitalization and/or cardiovascular death were a hazard ratio (HR) of 0.67 (95% confidence interval [CI]: 0.5 to 0.77), number needed to treat = 9 (7 to 14), and age extension of event-free survival +1.1 years (-0.1 to + 2.3 years). The corresponding metrics for EMPHASIS-HF (eplerenone vs. placebo in less severe HFrEF) were 0.64 (0.54 to 0.75), 14 (1 to 22), and +2.9 (1.2 to 4.5). In patients in PARADIGM-HF aged younger than 65 years, the metrics for sacubitril/valsartan versus enalapril were 0.77 (95% CI: 0.68 to 0.88), 23 (15 to 44), and +1.7 (0.6 to 2.8) years; for those aged 65 years or older, the metrics were 0.83 (95% CI: 0.73 to 0.94), 29 (17 to 83), and +0.9 (0.2 to 1.6) years, which provided evidence of a greater potential life extension in younger patients. Similar observations were found for lower risk patients.
Conclusions: RMST event-free (and overall) survival estimates provided a complementary means of evaluating the effect of therapy in relation to age and risk. They also provided a clinically useful metric that should be routinely reported and used to explain the potential long-term benefits of a given treatment, especially to younger and less symptomatic patients.
Keywords: restricted mean survival time; survival models; treatment effects; trials.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Author Disclosures Drs. Ferreira and Zannad are supported by ANR-15-RHU-0004 and ANR-15-IDEX-04-LUE. Drs. Jhund, Petrie, and McMurray are supported by the British Heart Foundation (grant RE/18/6/34217). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Comment in
-
Patient-Centered Measures of Treatment Benefit.JACC Heart Fail. 2020 Dec;8(12):996-998. doi: 10.1016/j.jchf.2020.09.003. Epub 2020 Oct 7. JACC Heart Fail. 2020. PMID: 33039449 No abstract available.
-
Bridging the Efficacy and Effectiveness Chasm.JACC Heart Fail. 2021 Jun;9(6):467-468. doi: 10.1016/j.jchf.2020.12.017. JACC Heart Fail. 2021. PMID: 34082901 No abstract available.
References
-
- Cox D.R. Regression models and life-tables. J Royal Stat Soc Series B (Methodological) 1972;34:187–220.
-
- Stensrud M.J., Aalen J.M., Aalen O.O., Valberg M. Limitations of hazard ratios in clinical trials. Eur Heart J. 2019;40:1378–1383. - PubMed
-
- Claggett B., Packer M., McMurray J.J. Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med. 2015;373:2289–2290. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

