Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor
- PMID: 33039659
- PMCID: PMC7749843
- DOI: 10.1016/j.bbi.2020.10.008
Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor
Abstract
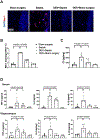
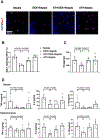
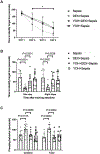
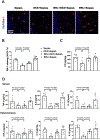
Sepsis-associated encephalopathy (SAE) is a significant clinical issue that is associated with increased mortality and cost of health care. Dexmedetomidine, an α2 adrenoceptor agonist that is used to provide sedation, has been shown to induce neuroprotection under various conditions. This study was designed to determine whether dexmedetomidine protects against SAE and whether α2 adrenoceptor plays a role in this protection. Six- to eight-week old CD-1 male mice were subjected to cecal ligation and puncture (CLP). They were treated with intraperitoneal injection of dexmedetomidine in the presence or absence of α2 adrenoceptor antagonists, atipamezole or yohimbine, or an α2A adrenoceptor antagonist, BRL-44408. Hippocampus and blood were harvested for measuring cytokines. Mice were subjected to Barnes maze and fear conditioning 14 days after CLP to evaluate their learning and memory. CLP significantly increased the proinflammatory cytokines including tumor necrosis factor α, interleukin (IL)-6 and IL-1β in the blood and hippocampus. CLP also increased the permeability of blood-brain barrier (BBB) and impaired learning and memory. These CLP detrimental effects were attenuated by dexmedetomidine. Intracerebroventricular application of atipamezole, yohimbine or BRL-44408 blocked the protection of dexmedetomidine on the brain but not on the systemic inflammation. Astrocytes but not microglia expressed α2A adrenoceptors. Microglial depletion did not abolish the protective effects of dexmedetomidine. These results suggest that dexmedetomidine reduces systemic inflammation, neuroinflammation, injury of BBB and cognitive dysfunction in septic mice. The protective effects of dexmedetomidine on the brain may be mediated by α2A adrenoceptors in the astrocytes.
Keywords: Astrocytes; Dexmedetomidine; Sepsis; Sepsis-associated encephalopathy; α2A adrenoceptor.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures







References
-
- Cobos-Puc LE, Aguayo-Morales H, Silva-Belmares Y, Gonzalez-Zavala MA, Centurion D, 2016. alpha2A-adrenoceptors, but not nitric oxide, mediate the peripheral cardiac sympatho-inhibition of moxonidine. Eur J Pharmacol 782 35–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

