Iron-mediated degradation of ribosomes under oxidative stress is attenuated by manganese
- PMID: 33040024
- PMCID: PMC7863898
- DOI: 10.1074/jbc.RA120.015025
Iron-mediated degradation of ribosomes under oxidative stress is attenuated by manganese
Abstract
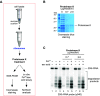
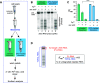
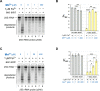
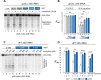
Protein biosynthesis is fundamental to cellular life and requires the efficient functioning of the translational machinery. At the center of this machinery is the ribosome, a ribonucleoprotein complex that depends heavily on Mg2+ for structure. Recent work has indicated that other metal cations can substitute for Mg2+, raising questions about the role different metals may play in the maintenance of the ribosome under oxidative stress conditions. Here, we assess ribosomal integrity following oxidative stress both in vitro and in cells to elucidate details of the interactions between Fe2+ and the ribosome and identify Mn2+ as a factor capable of attenuating oxidant-induced Fe2+-mediated degradation of rRNA. We report that Fe2+ promotes degradation of all rRNA species of the yeast ribosome and that it is bound directly to RNA molecules. Furthermore, we demonstrate that Mn2+ competes with Fe2+ for rRNA-binding sites and that protection of ribosomes from Fe2+-mediated rRNA hydrolysis correlates with the restoration of cell viability. Our data, therefore, suggest a relationship between these two transition metals in controlling ribosome stability under oxidative stress.
Keywords: RNA folding; cell viability; degradation of ribosomes; iron; iron metabolism; manganese; metals; oxidants; oxidative stress; ribosomal ribonucleic acid (rRNA); ribosome; ribosome structure; yeast.
© 2020 Smethurst et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures




References
-
- Weston, J. (2009) Biochemistry of magnesium. In PATAI'S Chemistry of Functional Groups (Rappoport, Z., ed) John Wiley & Sons, Chichester, UK
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

