Megalencephalic leukoencephalopathy with subcortical cysts 1 (MLC1) promotes glioblastoma cell invasion in the brain microenvironment
- PMID: 33040087
- PMCID: PMC7736299
- DOI: 10.1038/s41388-020-01503-9
Megalencephalic leukoencephalopathy with subcortical cysts 1 (MLC1) promotes glioblastoma cell invasion in the brain microenvironment
Abstract
Glioblastoma (GBM), or grade IV astrocytoma, is a malignant brain cancer that contains subpopulations of proliferative and invasive cells that coordinately drive primary tumor growth, progression, and recurrence after therapy. Here, we have analyzed functions for megalencephalic leukoencephalopathy with subcortical cysts 1 (Mlc1), an eight-transmembrane protein normally expressed in perivascular brain astrocyte end feet that is essential for neurovascular development and physiology, in the pathogenesis of GBM. We show that Mlc1 is expressed in human stem-like GBM cells (GSCs) and is linked to the development of primary and recurrent GBM. Genetically inhibiting MLC1 in GSCs using RNAi-mediated gene silencing results in diminished growth and invasion in vitro as well as impaired tumor initiation and progression in vivo. Biochemical assays identify the receptor tyrosine kinase Axl and its intracellular signaling effectors as important for MLC1 control of GSC invasive growth. Collectively, these data reveal key functions for MLC1 in promoting GSC growth and invasion, and suggest that targeting the Mlc1 protein or its associated signaling effectors may be a useful therapy for blocking tumor progression in patients with primary or recurrent GBM.
Conflict of interest statement
Figures
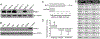






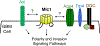
References
-
- Muir M, Gopakumar S, Traylor J, Lee S, Rao G. Glioblastoma multiforme: novel therapeutic targets. Expert Opin Ther Targets 2020: 1–10. - PubMed
-
- Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R et al. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020; 86: 102017. - PubMed
-
- Jhaveri N, Chen TC, Hofman FM. Tumor vasculature and glioma stem cells: Contributions to glioma progression. Cancer letters 2016; 380: 545–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

