Cyclooxygenase activity mediates colorectal cancer cell resistance to the omega-3 polyunsaturated fatty acid eicosapentaenoic acid
- PMID: 33040178
- PMCID: PMC7870614
- DOI: 10.1007/s00280-020-04157-2
Cyclooxygenase activity mediates colorectal cancer cell resistance to the omega-3 polyunsaturated fatty acid eicosapentaenoic acid
Erratum in
-
Correction to: Cyclooxygenase activity mediates colorectal cancer cell resistance to the omega‑3 polyunsaturated fatty acid eicosapentaenoic acid.Cancer Chemother Pharmacol. 2021 Jun;87(6):879-880. doi: 10.1007/s00280-020-04194-x. Cancer Chemother Pharmacol. 2021. PMID: 33200275 Free PMC article. No abstract available.
Abstract
Purpose: The naturally-occurring omega-3 polyunsaturated fatty acid eicosapentaenoic acid (EPA) is safe, well-tolerated and inexpensive, making it an attractive anti-cancer intervention. However, EPA has only modest anti-colorectal cancer (CRC) activity, when used alone. Both cyclooxygenase (COX) isoforms metabolise EPA and are over-expressed in CRC cells. We investigated whether COX inhibition increases the sensitivity of CRC cells to growth inhibition by EPA.
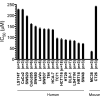
Methods: A panel of 18 human and mouse CRC cell lines was used to characterize the differential sensitivity of CRC cells to the growth inhibitory effects of EPA. The effect of CRISPR-Cas9 genetic deletion and pharmacological inhibition of COX-1 and COX-2 on the anti-cancer activity of EPA was determined using in vitro and in vivo models.
Results: Genetic ablation of both COX isoforms increased sensitivity of CT26 mouse CRC cells to growth inhibition by EPA in vitro and in vivo. The non-selective COX inhibitor aspirin and the selective COX-2 inhibitor celecoxib increased sensitivity of several human and mouse CRC cell lines to EPA in vitro. However, in a MC38 mouse CRC cell tumour model, with dosing that mirrored low-dose aspirin use in humans, thereby producing significant platelet COX-1 inhibition, there was ineffective intra-tumoral COX-2 inhibition by aspirin and no effect on EPA sensitivity of MC38 cell tumours.
Conclusion: Cyclooxygenase inhibition by non-steroidal anti-inflammatory drugs represents a therapeutic opportunity to augment the modest anti-CRC activity of EPA. However, intra-tumoral COX inhibition is likely to be critical for this drug-nutrient interaction and careful tissue pharmacodynamic profiling is required in subsequent pre-clinical and human studies.
Keywords: Aspirin; Cancer pharmacology; Celecoxib; Colorectal cancer; Cyclooxygenase; Drug metabolism; Eicosapentaenoic acid.
Conflict of interest statement
MAH has received honoraria from Bayer AG and SLA Pharma AG. MAH has previously received an unrestricted scientific grant from SLA Pharma AG unrelated to this work.
Figures





References
-
- Lai HT, de Oliveira Otto MC, Lemaitre RN, McKnight B, Song X, King IB, Chaves PH, Odden MC, Newman AB, Siscovick DS, Mozaffarian D. Serial circulating omega 3 polyunsaturated fatty acids and healthy ageing among older adults in the Cardiovascular Health Study: prospective cohort study. BMJ. 2018;363:k4067. doi: 10.1136/bmj.k4067. - DOI - PMC - PubMed
-
- Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM, Investigators R-I. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. doi: 10.1056/NEJMoa1812792. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

