Human papillomavirus vaccine to prevent cervical intraepithelial neoplasia in Japan: A nationwide case-control study
- PMID: 33040433
- PMCID: PMC7893998
- DOI: 10.1111/cas.14682
Human papillomavirus vaccine to prevent cervical intraepithelial neoplasia in Japan: A nationwide case-control study
Abstract
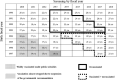
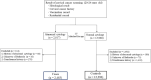
Cervical cancer remains among the most common cancers in women worldwide and can be prevented by vaccination. The Ministry of Health, Labour and Welfare of Japan suspended active recommendation of regular human papillomavirus (HPV) vaccines in 2013 because of various symptoms including chronic pain and motor impairment. This nationwide case-control study from April 2013 to March 2017 targeted women aged 20-24 years old at cervical screening. We compared HPV vaccination exposure between those with abnormal and normal cytology. Abnormal cytology was classified based on the results of histological test and we calculated the odds ratio (OR) and 95% confidence interval (CI) of the above endpoints and vaccination exposure using the conditional logistic regression model and estimated vaccine effectiveness using the formula (1 - OR) × 100. A total of 2483 cases and 12 296 controls (one-to-five matching) were eligible in 31 municipalities in Japan. The distribution of histological abnormalities among cases was 797 CIN1 (including dysplasia) (32.1%), 165 CIN2 (6.7%), 44 CIN3 (1.8%), and eight squamous cell carcinoma (SCC) (0.3%). The OR of HPV vaccination compared with no vaccination for abnormal cytology, CIN1+, CIN2+, and CIN3+ versus controls was 0.42 (95% CI, 0.34-0.50), 0.42 (95% CI, 0.31-0.58), 0.25 (95% CI, 0.12-0.54), and 0.19 (95% CI, 0.03-1.15), respectively, equating to a vaccine effectiveness of 58.5%, 57.9%, 74.8%, and 80.9%, respectively. Eight patients had SCC, none was vaccinated. This nationwide case-control study in Japan demonstrated a substantial risk reduction in abnormal cytology and CIN among women who did versus those who did not receive HPV vaccination.
Keywords: HPV vaccine; case-control study; cervical intraepithelial neoplasia; human papillomavirus; vaccine effectiveness.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
AY, YU, MH, and TE received a lecture fee and YU received a research grant (J550703673) from Merck Sharp & Dohme (MSD).
Figures
References
-
- Walboomers JMM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12‐19. - PubMed
-
- Munoz N, Kjaer S, Sigurdsson K, et al. Impact of human papillomavirus (HPV)‐6/11/16/18 vaccine on all HPV‐associated genital diseases in young women. JNCI. 2010;102:325‐339. - PubMed
-
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)‐16/18 A04‐adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double‐blind, randomized study in young women. Lancet. 2009;374:301‐314. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

