Isolation and Differentiation of Amniotic Membrane Stem Cells Into Keratinocytes
- PMID: 33040596
- PMCID: PMC7784561
- DOI: 10.1177/0963689720964381
Isolation and Differentiation of Amniotic Membrane Stem Cells Into Keratinocytes
Abstract
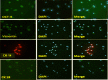
The human amniotic membrane is a highly abundant and readily available tissue that may be useful for regenerative medicine and cell therapy. The amniotic membrane stem cells can differentiate into multiple cell lineages; they have low immunogenicity and anti-inflammatory functions. This research aims to examine the protocols for the isolation of human amniotic membrane stem cells, including their phenotypic characterization and in vitro potential for differentiation toward keratinocytes. Human placentas were obtained from selected cesarean-sectioned births. We isolated amniotic stem cells by trypsin and collagenase B digestion and centrifuged with Percoll. After monolayer expansion of adherent cells, the cells were characterized by immunocytology with octamer-binding transcription factor 4 and differentiated into keratinocytes by treating the cells with insulin, hydrocortisone, BMP-4, and vitamin C. Protocol for isolation of stem cells from amniotic membrane has high efficiency. Differentiation markers of stem cells into keratinocytes, such as vimentin, cytokeratin (CK) 14, and CK19, were determined by reverse transcription-polymerase chain reaction increase over time in culture. Stem cells isolated from the amniotic membrane can differentiate into keratinocytes. It has opened the prospect of using stem cells to regenerate skin and clinical applications.
Keywords: amniotic membrane; cytokeratin; differentiation; keratinocytes; stem cells.
Conflict of interest statement
Figures


References
-
- Zhao P, Ise H, Hongo M, Ota M, Konishi I, Nikaido T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation. 2005;79(5):528–535. - PubMed
-
- Yuge I., Takumi Y, Koyabu K, Hashimoto S, Takashima S, Fukuyama T, Nikaido T, Usami S. Transplanted human amniotic epithelial cells express connexin 26 and Na-K-adenosine triphosphatase in the inner ear. Transplantation. 2004;77(9):1452–1454. - PubMed
-
- Young M J, Borras T, Walter M, Ritch R. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11(9-10):1599–1610. - PubMed
-
- Yang L, Shirakata Y, Shudou M, Dai X, Tokumaru S, Hirakawa S, Sayama K, Hamuro J, Hashimoto K. New skin-equivalent model from de-epithelialized amnion membrane. Cell Tissue Res. 2006;326(1):69–77. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

