Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine
- PMID: 33041105
- PMCID: PMC7545304
- DOI: 10.1016/j.vaccine.2020.09.079
Protection against herpes simplex virus type 2 infection in a neonatal murine model using a trivalent nucleoside-modified mRNA in lipid nanoparticle vaccine
Abstract
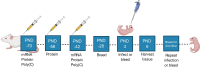
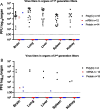
Neonatal herpes is a dreaded complication of genital herpes infection in pregnancy. We recently compared two vaccine platforms for preventing genital herpes in female mice and guinea pigs and determined that HSV-2 glycoproteins C, D and E expressed using nucleoside-modified mRNA in lipid nanoparticles provided better protection than the same antigens produced as baculovirus proteins and administered with CpG and alum. Here we evaluated mRNA and protein immunization for protection against neonatal herpes. Female mice were immunized prior to mating and newborns were infected intranasally with HSV-2. IgG binding and neutralizing antibody levels in mothers and newborns were comparable using the mRNA or protein vaccines. Both vaccines protected first and second litter newborns against disseminated infection based on virus titers in multiple organs. We conclude that both vaccines are efficacious at preventing neonatal herpes, which leaves the mRNA vaccine as our preferred candidate based on better protection against genital herpes.
Keywords: Genital herpes; Herpes simplex virus type 2; Maternal antibody; Neonatal herpes; Neutralizing antibody; Trivalent nucleoside-modified mRNA vaccine in lipid nanoparticles.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: HMF, SA, GHC and DW are inventors on patents held by the University of Pennsylvania for protein (HMF, SA) and mRNA (HMF, SA, GHC, DW) vaccines for genital herpes. NP is also named on a patent describing use of nucleoside-modified mRNA-LNP as a vaccine platform. The authors have disclosed their interests fully to the University of Pennsylvania, and have in place an approved plan for managing any potential conflicts arising from licensing of the patents.
Figures

Similar articles
-
A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1.Viruses. 2023 Jun 30;15(7):1483. doi: 10.3390/v15071483. Viruses. 2023. PMID: 37515169 Free PMC article.
-
Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes.J Virol. 2014 Aug;88(15):8421-32. doi: 10.1128/JVI.01130-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829358 Free PMC article.
-
Herpes simplex virus type 2 trivalent protein vaccine containing glycoproteins C, D and E protects guinea pigs against HSV-1 genital infection.Hum Vaccin Immunother. 2020 Sep 1;16(9):2109-2113. doi: 10.1080/21645515.2020.1749509. Epub 2020 Apr 29. Hum Vaccin Immunother. 2020. PMID: 32347775 Free PMC article.
-
Vaccines to prevent genital herpes.Transl Res. 2020 Jun;220:138-152. doi: 10.1016/j.trsl.2020.03.004. Epub 2020 Mar 16. Transl Res. 2020. PMID: 32272093 Free PMC article. Review.
-
An mRNA vaccine to prevent genital herpes.Transl Res. 2022 Apr;242:56-65. doi: 10.1016/j.trsl.2021.12.006. Epub 2021 Dec 23. Transl Res. 2022. PMID: 34954087 Free PMC article. Review.
Cited by
-
Lipid-nanoparticle-encapsulated mRNA vaccines induce protective memory CD8 T cells against a lethal viral infection.Mol Ther. 2021 Sep 1;29(9):2769-2781. doi: 10.1016/j.ymthe.2021.05.011. Epub 2021 May 14. Mol Ther. 2021. PMID: 33992803 Free PMC article.
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
-
Assessment of a quadrivalent nucleoside-modified mRNA vaccine that protects against group 2 influenza viruses.Proc Natl Acad Sci U S A. 2022 Nov 8;119(45):e2206333119. doi: 10.1073/pnas.2206333119. Epub 2022 Nov 2. Proc Natl Acad Sci U S A. 2022. PMID: 36322769 Free PMC article.
-
An updated review of HSV-1 infection-associated diseases and treatment, vaccine development, and vector therapy application.Virulence. 2024 Dec;15(1):2425744. doi: 10.1080/21505594.2024.2425744. Epub 2024 Nov 13. Virulence. 2024. PMID: 39508503 Free PMC article. Review.
-
mRNA vaccines: Past, present, future.Asian J Pharm Sci. 2022 Jul;17(4):491-522. doi: 10.1016/j.ajps.2022.05.003. Epub 2022 Jun 30. Asian J Pharm Sci. 2022. PMID: 36105317 Free PMC article. Review.
References
-
- Prober C.G., Sullender W.M., Yasukawa L.L., Au D.S., Yeager A.S., Arvin A.M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. New England J Med. 1987;316:240–244. - PubMed
-
- Zaman K., Roy E., Arifeen S.E., Rahman M., Raqib R., Wilson E. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–1564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

