The Revolution in Pediatric Drug Development and Drug Use: Therapeutic Orphans No More
- PMID: 33041711
- PMCID: PMC7541025
- DOI: 10.5863/1551-6776-25.7.565
The Revolution in Pediatric Drug Development and Drug Use: Therapeutic Orphans No More
Abstract
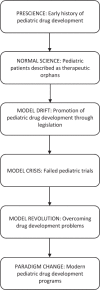
This lecture was given by Dr. Burckart in association with presentation of the 2014 Sumner J. Yaffe Lifetime Achievement Award in Pediatric Pharmacology and Therapeutics, which is selected by the Pediatric Pharmacy Association. Multiple factors make conducting drug studies in the pediatric population difficult, resulting in a historic lack of information surrounding safe and efficacious drug dosing in children. The paradigm in pediatric drug development has shifted from normal science being that children are therapeutic orphans in the drug development system, to a model drift caused by pediatric legislation, to a model crisis caused by failed pediatric drug development trials, to finally a model revolution that includes pediatric patients routinely in drug development. Major regulatory actions and the accumulation of scientific evidence has created an environment where clinicians can expect properly labeled drug usage information for the pediatric population.
Keywords: Food and Drug Administration; Sumner J. Yaffe; drug approval; drug development; pediatric pharmacology.
Copyright Pediatric Pharmacy Association. All rights reserved. For permissions, email: mhelms@pediatricpharmacy.org 2020.
Conflict of interest statement
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors received no financial support for the research, authorship, and/or publication of this article.
Figures
References
-
- Kuhn TS. The Structure of Scientific Revolutions. Chicago, IL: University of Chicago Press; 1962.
-
- Vanchieri C, Butler AS, Knutsen A. Addressing the Barriers to Pediatric Drug Development: Workshop Summary. Washington, DC: The National Academies Press; 2008. - PubMed
-
- Pure Food and Drug Act, 21 U.S.C. §§1–15. 1906.
LinkOut - more resources
Full Text Sources