Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena
- PMID: 33041830
- PMCID: PMC7527880
- DOI: 10.3389/fphar.2020.585888
Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena
Abstract
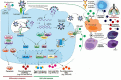
Coronavirus disease 2019 (COVID-19), an acute onset pneumonia caused by a novel Betacoronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in the Wuhan City of China in December 2019 and evolved into a global pandemic. To date, there are no proven drugs or vaccines against this virus. Hence, the situation demands an urgent need to explore all potential therapeutic strategies that can be made available to prevent the disease progression and improve patient outcomes. In absence of clinically proven treatment guidelines, several repurposed drugs and investigational agents are currently being evaluated in clinical trials for their probable benefits in the treatment of COVID-19. These include antivirals (remdesivir, lopinavir/ritonavir, umifenovir, and favipiravir), interferon, antimalarials (chloroquine/hydroxychloroquine), antiparasitic drugs (ivermectin and nitazoxanide), biologics (monoclonal antibodies and interleukin receptor antagonist), cellular therapies (mesenchymal stem cells and natural killer cells), convalescent plasma, and cytokine adsorber. Though several observational studies have claimed many of these agents to be effective based on their in vitro activities and extrapolated evidence from SARS and Middle East respiratory syndrome (MERS) epidemics, the currently available data remains inconclusive because of ill-defined patient selection criteria, small sample size, lack of concurrent controls, and use of intermediary outcomes instead of patient-relevant outcomes. Moreover, there is a need to clearly define the patient populations who warrant therapy and also the timing of initiation of treatment. Understanding the disease pathology responsible for the clinical manifestations of COVID-19 is imperative to identify the potential targets for drug development. This review explains the pathophysiology of COVID-19 and summarizes the potential treatment candidates, which can provide guidance in developing effective therapeutic strategies.
Keywords: anti-inflammatory; antiviral; coronavirus disease 2019; drug repurposing; immunotherapy; pathophysiology; severe acute respiratory syndrome coronavirus 2.
Copyright © 2020 Samaddar, Grover and Nag.
Figures

References
-
- Atluri S., Manchikanti L., Hirsch J. A. (2020). Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician 23, E71–E83. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

