New Insights Into Heat Shock Protein 90 in the Pathogenesis of Pulmonary Arterial Hypertension
- PMID: 33041844
- PMCID: PMC7522509
- DOI: 10.3389/fphys.2020.01081
New Insights Into Heat Shock Protein 90 in the Pathogenesis of Pulmonary Arterial Hypertension
Abstract
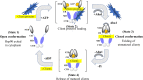
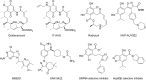
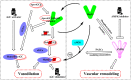
Pulmonary arterial hypertension (PAH) is a multifactorial and progressive disorder. This disease is characterized by vasoconstriction and vascular remodeling, which results in increased pulmonary artery pressure and pulmonary vascular resistance. Although extensive studies have been carried out to understand the etiology, it is still unclear what intracellular factors contribute and integrate these pathological features. Heat shock protein 90 (Hsp90), a ubiquitous and essential molecular chaperone, is involved in the maturation of many proteins. An increasing number of studies have revealed direct connections between abnormal Hsp90 expression and cellular factors related to PAH, such as soluble guanylate cyclase and AMP-activated protein kinase. These studies suggest that the Hsp90 regulatory network is a major predictor of poor outcomes, providing novel insights into the pathogenesis of PAH. For the first time, this review summarizes the interplay between the Hsp90 dysregulation and different proteins involved in PAH development, shedding novel insights into the intrinsic pathogenesis and potentially novel therapeutic strategies for this devastating disease.
Keywords: AMP-activated protein kinase; heat shock protein 90; novel therapeutic options; pathogenesis; pulmonary arterial hypertension; soluble guanylate cyclase.
Copyright © 2020 Hu, Zhao, Liu and Li.
Figures


Similar articles
-
Inhibition of heat shock protein 90 improves pulmonary arteriole remodeling in pulmonary arterial hypertension.Oncotarget. 2016 Aug 23;7(34):54263-54273. doi: 10.18632/oncotarget.10855. Oncotarget. 2016. PMID: 27472464 Free PMC article.
-
Mitochondrial HSP90 Accumulation Promotes Vascular Remodeling in Pulmonary Arterial Hypertension.Am J Respir Crit Care Med. 2018 Jul 1;198(1):90-103. doi: 10.1164/rccm.201708-1751OC. Am J Respir Crit Care Med. 2018. PMID: 29394093 Free PMC article.
-
[Novel concepts in the pathobiology of pulmonary arterial hypertension].Dtsch Med Wochenschr. 2008 Oct;133 Suppl 6:S167-9. doi: 10.1055/s-0028-1091229. Epub 2008 Sep 23. Dtsch Med Wochenschr. 2008. PMID: 18814087 Review. German.
-
Cellular interplay in pulmonary arterial hypertension: implications for new therapies.Biochim Biophys Acta. 2014 May;1843(5):885-93. doi: 10.1016/j.bbamcr.2014.01.030. Epub 2014 Jan 31. Biochim Biophys Acta. 2014. PMID: 24491811 Review.
-
Expression and function of soluble guanylate cyclase in pulmonary arterial hypertension.Eur Respir J. 2008 Oct;32(4):881-91. doi: 10.1183/09031936.00114407. Epub 2008 Jun 11. Eur Respir J. 2008. PMID: 18550612
Cited by
-
Emerging insights into pulmonary hypertension: the potential role of mitochondrial dysfunction and redox homeostasis.Mol Cell Biochem. 2025 Mar;480(3):1407-1429. doi: 10.1007/s11010-024-05096-9. Epub 2024 Sep 10. Mol Cell Biochem. 2025. PMID: 39254871 Review.
-
Stem cell therapy for pulmonary arterial hypertension: An update.J Heart Lung Transplant. 2022 Jun;41(6):692-703. doi: 10.1016/j.healun.2022.02.020. Epub 2022 Mar 6. J Heart Lung Transplant. 2022. PMID: 35341679 Free PMC article. Review.
-
The Potential Application and Promising Role of Targeted Therapy in Pulmonary Arterial Hypertension.Biomedicines. 2022 Jun 15;10(6):1415. doi: 10.3390/biomedicines10061415. Biomedicines. 2022. PMID: 35740436 Free PMC article. Review.
References
-
- Benza R. L., Miller D. P., Gomberg-Maitland M., Frantz R. P., Foreman A. J., Coffey C. S., et al. (2010). Predicting survival in pulmonary arterial hypertension: insights from the Registry to evaluate early and long-term pulmonary arterial hypertension disease management (REVEAL). Circulation 122 164–172. 10.1161/CIRCULATIONAHA.109.898122 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

