The Emerging Role of RHOT1/Miro1 in the Pathogenesis of Parkinson's Disease
- PMID: 33041957
- PMCID: PMC7523470
- DOI: 10.3389/fneur.2020.00587
The Emerging Role of RHOT1/Miro1 in the Pathogenesis of Parkinson's Disease
Abstract
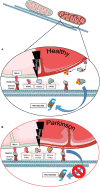
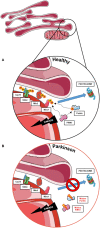
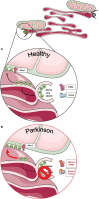
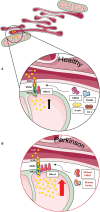
The expected increase in prevalence of Parkinson's disease (PD) as the most common neurodegenerative movement disorder over the next years underscores the need for a better understanding of the underlying molecular pathogenesis. Here, first insights provided by genetics over the last two decades, such as dysfunction of molecular and organellar quality control, are described. The mechanisms involved relate to impaired intracellular calcium homeostasis and mitochondrial dynamics, which are tightly linked to the cross talk between the endoplasmic reticulum (ER) and mitochondria. A number of proteins related to monogenic forms of PD have been mapped to these pathways, i.e., PINK1, Parkin, LRRK2, and α-synuclein. Recently, Miro1 was identified as an important player, as several studies linked Miro1 to mitochondrial quality control by PINK1/Parkin-mediated mitophagy and mitochondrial transport. Moreover, Miro1 is an important regulator of mitochondria-ER contact sites (MERCs), where it acts as a sensor for cytosolic calcium levels. The involvement of Miro1 in the pathogenesis of PD was recently confirmed by genetic evidence based on the first PD patients with heterozygous mutations in RHOT1/Miro1. Patient-based cellular models from RHOT1/Miro1 mutation carriers showed impaired calcium homeostasis, structural alterations of MERCs, and increased mitochondrial clearance. To account for the emerging role of Miro1, we present a comprehensive overview focusing on the role of this protein in PD-related neurodegeneration and highlighting new developments in our understanding of Miro1, which provide new avenues for neuroprotective therapies for PD patients.
Keywords: Miro1; Parkinson's disease; calcium signaling; mitochondrial dynamics; mitophagy.
Copyright © 2020 Grossmann, Berenguer-Escuder, Chemla, Arena and Krüger.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

