Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health?
- PMID: 33042016
- PMCID: PMC7516342
- DOI: 10.3389/fendo.2020.00655
Mechanisms of Endothelial Dysfunction in Pre-eclampsia and Gestational Diabetes Mellitus: Windows Into Future Cardiometabolic Health?
Abstract
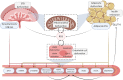
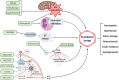
Placental insufficiency and adipose tissue dysregulation are postulated to play key roles in the pathophysiology of both pre-eclampsia (PE) and gestational diabetes mellitus (GDM). A dysfunctional release of deleterious signaling motifs can offset an increase in circulating oxidative stressors, pro-inflammatory factors and various cytokines. It has been previously postulated that endothelial dysfunction, instigated by signaling from endocrine organs such as the placenta and adipose tissue, may be a key mediator of the vasculopathy that is evident in both adverse obstetric complications. These signaling pathways also have significant effects on long term maternal cardiometabolic health outcomes, specifically cardiovascular disease, hypertension, and type II diabetes. Recent studies have noted that both PE and GDM are strongly associated with lower maternal flow-mediated dilation, however the exact pathways which link endothelial dysfunction to clinical outcomes in these complications remains in question. The current diagnostic regimen for both PE and GDM lacks specificity and consistency in relation to clinical guidelines. Furthermore, current therapeutic options rely largely on clinical symptom control such as antihypertensives and insulin therapy, rather than that of early intervention or prophylaxis. A better understanding of the pathogenic origin of these obstetric complications will allow for more targeted therapeutic interventions. In this review we will explore the complex signaling relationship between the placenta and adipose tissue in PE and GDM and investigate how these intricate pathways affect maternal endothelial function and, hence, play a role in acute pathophysiology and the development of future chronic maternal health outcomes.
Keywords: GDM; PE; adiposity; endothelial dysfunction; mitochondria; oxidative stress; therapeutics.
Copyright © 2020 McElwain, Tuboly, McCarthy and McCarthy.
Figures
Similar articles
-
Markers of endothelial cell dysfunction are increased in human omental adipose tissue from women with pre-existing maternal obesity and gestational diabetes.Metabolism. 2014 Jun;63(6):860-73. doi: 10.1016/j.metabol.2014.03.007. Epub 2014 Mar 14. Metabolism. 2014. PMID: 24684825
-
Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus.J Physiol. 2021 Feb;599(4):1291-1305. doi: 10.1113/JP280593. Epub 2020 Nov 15. J Physiol. 2021. PMID: 33135816
-
Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies.J Endocrinol. 2005 Sep;186(3):457-65. doi: 10.1677/joe.1.06227. J Endocrinol. 2005. PMID: 16135665
-
Implications of pregnancy on cardiometabolic disease risk: preeclampsia and gestational diabetes.Am J Physiol Cell Physiol. 2024 Sep 1;327(3):C646-C660. doi: 10.1152/ajpcell.00293.2024. Epub 2024 Jul 16. Am J Physiol Cell Physiol. 2024. PMID: 39010840 Review.
-
Role of adipose tissue in regulating fetal growth in gestational diabetes mellitus.Placenta. 2020 Dec;102:39-48. doi: 10.1016/j.placenta.2020.05.006. Epub 2020 May 25. Placenta. 2020. PMID: 33218577 Review.
Cited by
-
A new classification method for gestational diabetes mellitus: a study on the relationship between abnormal blood glucose values at different time points in oral glucose tolerance test and adverse maternal and neonatal outcomes in pregnant women with gestational diabetes mellitus.AJOG Glob Rep. 2024 Aug 15;4(4):100390. doi: 10.1016/j.xagr.2024.100390. eCollection 2024 Nov. AJOG Glob Rep. 2024. PMID: 39309607 Free PMC article.
-
MicroRNAs in the Pathogenesis of Preeclampsia-A Case-Control In Silico Analysis.Curr Issues Mol Biol. 2024 Apr 17;46(4):3438-3459. doi: 10.3390/cimb46040216. Curr Issues Mol Biol. 2024. PMID: 38666946 Free PMC article.
-
Investigating the Role of Skin Autofluorescence in Gestational Diabetes Mellitus: A Systematic Review.Int J Mol Sci. 2025 Mar 26;26(7):3022. doi: 10.3390/ijms26073022. Int J Mol Sci. 2025. PMID: 40243644 Free PMC article.
-
Plasma from hypertensive pregnancy patients induce endothelial dysfunction even under atheroprotective shear stress.Sci Rep. 2025 Feb 8;15(1):4675. doi: 10.1038/s41598-025-88902-8. Sci Rep. 2025. PMID: 39920219 Free PMC article.
-
Gestational Diabetes Mellitus and Maternal Immune Dysregulation: What We Know So Far.Int J Mol Sci. 2021 Apr 20;22(8):4261. doi: 10.3390/ijms22084261. Int J Mol Sci. 2021. PMID: 33923959 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

