Molecular Footprints of the Immune Assault on Pancreatic Beta Cells in Type 1 Diabetes
- PMID: 33042023
- PMCID: PMC7522353
- DOI: 10.3389/fendo.2020.568446
Molecular Footprints of the Immune Assault on Pancreatic Beta Cells in Type 1 Diabetes
Abstract
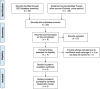
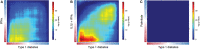
Type 1 diabetes (T1D) is a chronic disease caused by the selective destruction of the insulin-producing pancreatic beta cells by infiltrating immune cells. We presently evaluated the transcriptomic signature observed in beta cells in early T1D and compared it with the signatures observed following in vitro exposure of human islets to inflammatory or metabolic stresses, with the aim of identifying "footprints" of the immune assault in the target beta cells. We detected similarities between the beta cell signatures induced by cytokines present at different moments of the disease, i.e., interferon-α (early disease) and interleukin-1β plus interferon-γ (later stages) and the beta cells from T1D patients, identifying biological process and signaling pathways activated during early and late stages of the disease. Among the first responses triggered on beta cells was an enrichment in antiviral responses, pattern recognition receptors activation, protein modification and MHC class I antigen presentation. During putative later stages of insulitis the processes were dominated by T-cell recruitment and activation and attempts of beta cells to defend themselves through the activation of anti-inflammatory pathways (i.e., IL10, IL4/13) and immune check-point proteins (i.e., PDL1 and HLA-E). Finally, we mined the beta cell signature in islets from T1D patients using the Connectivity Map, a large database of chemical compounds/drugs, and identified interesting candidates to potentially revert the effects of insulitis on beta cells.
Keywords: RNA-sequencing; beta cells; inflammation; insulitis; interferon; pancreatic islets; therapeutics; type 1 diabetes.
Copyright © 2020 Colli, Szymczak and Eizirik.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

