Fumarase From Cyanidioschyzon merolae Stably Shows High Catalytic Activity for Fumarate Hydration Under High Temperature Conditions
- PMID: 33042040
- PMCID: PMC7525151
- DOI: 10.3389/fmicb.2020.560894
Fumarase From Cyanidioschyzon merolae Stably Shows High Catalytic Activity for Fumarate Hydration Under High Temperature Conditions
Abstract
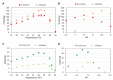
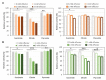
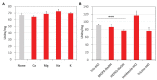
Fumarases (Fums) catalyze the reversible reaction converting fumarate to l-malate. There are two kinds of Fums: Class І and ІІ. Thermostable Class ІІ Fums, from mesophilic microorganisms, are utilized for industrial l-malate production. However, the low thermostability of these Fums is a limitation in industrial l-malate production. Therefore, an alternative Class ІІ Fum that shows high activity and thermostability is required to overcome this drawback. Thermophilic microalgae and cyanobacteria can use carbon dioxide as a carbon source and are easy to cultivate. Among them, Cyanidioschyzon merolae and Thermosynechococcus elongatus are model organisms to study cell biology and structural biology, respectively. We biochemically analyzed Class ІІ Fums from C. merolae (CmFUM) and T. elongatus (TeFum). Both CmFUM and TeFum preferentially catalyzed fumarate hydration. The catalytic activity of CmFUM for fumarate hydration in the optimum conditions (52°C and pH 7.5) is higher compared to those of Class ІІ Fums from other organisms and TeFum. Thermostability tests of CmFUM revealed that CmFUM showed higher thermostability than those of Class ІІ Fums from other microorganisms. The yield of l-malate obtained from fumarate hydration catalyzed by CmFUM was 75-81%. In summary, CmFum has suitable properties for efficient l-malate production.
Keywords: cyanobacteria; fumarase; l-malate; microalgae; tricarboxylic acid cycle.
Copyright © 2020 Ito, Iwazumi, Sukigara and Osanai.
Figures





Similar articles
-
Immobilization of fumarase from thermophilic eukaryotic red alga Cyanidioschyzon merolae on ceramic carrier.J Gen Appl Microbiol. 2024 Sep 4;70(2). doi: 10.2323/jgam.2024.02.003. Epub 2024 Feb 29. J Gen Appl Microbiol. 2024. PMID: 38417903
-
Identification and characterization of a novel fumarase gene by metagenome expression cloning from marine microorganisms.Microb Cell Fact. 2010 Nov 23;9:91. doi: 10.1186/1475-2859-9-91. Microb Cell Fact. 2010. PMID: 21092234 Free PMC article.
-
Identification of a novel fumarase C from Streptomyces lividans TK54 as a good candidate for L-malate production.Mol Biol Rep. 2014 Jan;41(1):497-504. doi: 10.1007/s11033-013-2885-8. Epub 2013 Dec 5. Mol Biol Rep. 2014. PMID: 24307253
-
Purification and catalytic properties of "thermostable" fumarase from Bacillus stearothermophilus NU-10 and Thermus X-1.Experientia Suppl. 1976;26:207-22. doi: 10.1007/978-3-0348-7675-9_17. Experientia Suppl. 1976. PMID: 939272
-
Biological production of L-malate: recent advances and future prospects.World J Microbiol Biotechnol. 2017 Dec 6;34(1):6. doi: 10.1007/s11274-017-2349-8. World J Microbiol Biotechnol. 2017. PMID: 29214355 Review.
Cited by
-
Construction and Application of a Multienzyme System for Synthesis of L-malate.Appl Biochem Biotechnol. 2024 Dec;196(12):8965-8979. doi: 10.1007/s12010-024-05026-x. Epub 2024 Aug 1. Appl Biochem Biotechnol. 2024. PMID: 39088025
-
Citrate synthase from Cyanidioschyzon merolae exhibits high oxaloacetate and acetyl-CoA catalytic efficiency.Plant Mol Biol. 2023 Mar;111(4-5):429-438. doi: 10.1007/s11103-023-01335-7. Epub 2023 Mar 8. Plant Mol Biol. 2023. PMID: 36884198
-
Biochemical Properties of β-Amylase from Red Algae and Improvement of Its Thermostability through Immobilization.ACS Omega. 2022 Oct 3;7(41):36195-36205. doi: 10.1021/acsomega.2c03315. eCollection 2022 Oct 18. ACS Omega. 2022. PMID: 36278071 Free PMC article.
-
Superior thermostability and divalent cation sensitivity of isoamylase CMI294C from Cyanidioschyzon merolae.Plant Mol Biol. 2025 Jul 31;115(4):99. doi: 10.1007/s11103-025-01623-4. Plant Mol Biol. 2025. PMID: 40745388 Free PMC article.
References
-
- Chibata I., Tosa T., Yamamoto K. (1987). Production of l-malic acid by immobilized microbial cells. Methods Enzymol. 136, 455–463. 10.1016/S0076-6879(87)36043-4 - DOI
LinkOut - more resources
Full Text Sources

