Probiotics, Prebiotics, Synbiotics, and Paraprobiotics as a Therapeutic Alternative for Intestinal Mucositis
- PMID: 33042054
- PMCID: PMC7527409
- DOI: 10.3389/fmicb.2020.544490
Probiotics, Prebiotics, Synbiotics, and Paraprobiotics as a Therapeutic Alternative for Intestinal Mucositis
Abstract
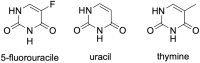
Intestinal mucositis, a cytotoxic side effect of the antineoplastic drug 5-fluorouracil (5-FU), is characterized by ulceration, inflammation, diarrhea, and intense abdominal pain, making it an important issue for clinical medicine. Given the seriousness of the problem, therapeutic alternatives have been sought as a means to ameliorate, prevent, and treat this condition. Among the alternatives available to address this side effect of treatment with 5-FU, the most promising has been the use of probiotics, prebiotics, synbiotics, and paraprobiotics. This review addresses the administration of these "biotics" as a therapeutic alternative for intestinal mucositis caused by 5-FU. It describes the effects and benefits related to their use as well as their potential for patient care.
Keywords: chemotherapy; intestinal inflammation; lactic acid bacteria; mucosite; treatment.
Copyright © 2020 Batista, da Silva, de Jesus, Coelho-Rocha, Barroso, Tavares, Azevedo, Mancha-Agresti and Drumond.
Figures

References
-
- Acurcio L. B., Sandes S. H. C., Bastos R. W., Sant’anna F. M., Pedroso S. H. S. P., Reis D. C., et al. (2017). Milk fermented by Lactobacillus species from Brazilian artisanal cheese protect germ-free-mice against Salmonella typhimurium infection. Benef. Microbes 8 579–588. 10.3920/BM2016.0163 - DOI - PubMed
-
- Aguilar-Toalá J. E., Garcia-Varela R., Garcia H. S., Mata-Haro V., González-Córdova A. F., Vallejo-Cordoba B., et al. (2018). Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 75 105–114. 10.1016/j.tifs.2018.03.009 - DOI
-
- Aliakbarpour H. R., Chamani M., Rahimi G., Sadeghi A. A., Qujeq D. (2012). The Bacillus subtilis and Lactic Acid Bacteria Probiotics Influences Intestinal Mucin Gene Expression, Histomorphology and Growth Performance in Broilers. Asian Austr. J. Anim. Sci. 25 1285–1293. 10.5713/ajas.2012.12110 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

