Colorectal Cancer Immunotherapy: Options and Strategies
- PMID: 33042104
- PMCID: PMC7530194
- DOI: 10.3389/fimmu.2020.01624
Colorectal Cancer Immunotherapy: Options and Strategies
Abstract
Colorectal cancer is the third most common cancer in the world with increasing incidence and mortality rates globally. Standard treatments for colorectal cancer have always been surgery, chemotherapy and radiotherapy which may be used in combination to treat patients. However, these treatments have many side effects due to their non-specificity and cytotoxicity toward any cells including normal cells that are growing and dividing. Furthermore, many patients succumb to relapse even after a series of treatments. Thus, it is crucial to have more alternative and effective treatments to treat CRC patients. Immunotherapy is one of the new alternatives in cancer treatment. The strategy is to utilize patients' own immune systems in combating the cancer cells. Cancer immunotherapy overcomes the issue of specificity which is the major problem in chemotherapy and radiotherapy. The normal cells with no cancer antigens are not affected. The outcomes of some cancer immunotherapy have been astonishing in some cases, but some which rely on the status of patients' own immune systems are not. Those patients who responded well to cancer immunotherapy have a better prognostic and better quality of life.
Keywords: FDA; T-cells; antibodies; colorectal carcinoma; cytokines; immunotherapy; treatments.
Copyright © 2020 Johdi and Sukor.
Figures

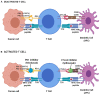
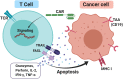
References
-
- Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. (2019). Available online at: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact... (accessed September 21, 2019).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

