Challenges for the Newborn Following Influenza Virus Infection and Prospects for an Effective Vaccine
- PMID: 33042150
- PMCID: PMC7524958
- DOI: 10.3389/fimmu.2020.568651
Challenges for the Newborn Following Influenza Virus Infection and Prospects for an Effective Vaccine
Abstract
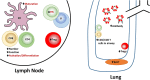
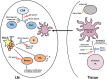
Newborns are at significantly increased risk of severe disease following infection with influenza virus. This is the collective result of their naïve status, altered immune responsiveness, and the lack of a vaccine that is effective in these individuals. Numerous studies have revealed impairments in both the innate and adaptive arms of the immune system of newborns. The consequence of these alterations is a quantitative and qualitative decrease in both antibody and T cell responses. This review summarizes the hurdles newborns experience in mounting an effective response that can clear influenza virus and limit disease following infection. In addition, the challenges, as well as the opportunities, for developing vaccines that can elicit protective responses in these at risk individuals are discussed.
Keywords: adjuvant; antibody; influenza; newborn; vaccine.
Copyright © 2020 Alexander-Miller.
Figures

References
-
- Thompson MG, Levine MZ, Bino S, Hunt DR, Al-Sanouri TM, Simoes EAF, et al. . Underdetection of laboratory-confirmed influenza-associated hospital admissions among infants: a multicentre, prospective study. Lancet Child Adolesc Health. (2019) 3:781–94. 10.1016/S2352-4642(19)30246-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

