Centrally Acting Angiotensin-Converting Enzyme Inhibitor Suppresses Type I Interferon Responses and Decreases Inflammation in the Periphery and the CNS in Lupus-Prone Mice
- PMID: 33042154
- PMCID: PMC7522287
- DOI: 10.3389/fimmu.2020.573677
Centrally Acting Angiotensin-Converting Enzyme Inhibitor Suppresses Type I Interferon Responses and Decreases Inflammation in the Periphery and the CNS in Lupus-Prone Mice
Abstract
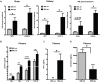
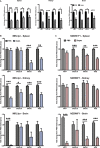
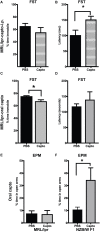
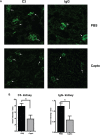
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by multi-organ damage. Neuropsychiatric lupus (NPSLE) is one of the most common manifestations of human SLE, often causing depression. Interferon-α (IFNα) is a central mediator in disease pathogenesis. Administration of IFNα to patients with chronic viral infections or cancers causes depressive symptoms. Angiotensin-converting enzyme (ACE) is part of the kallikrein-kinin/renin-angiotensin (KKS/RAS) system that regulates many physiological processes, including inflammation, and brain functions. It is known that ACE degrades bradykinin (BK) into inactive peptides. We have previously shown in an in vitro model of mouse bone-marrow-derived dendritic cells (BMDC) and human peripheral blood mononuclear cells that captopril (a centrally acting ACE inhibitor-ACEi) suppressed Type I IFN responsive gene (IRG) expression. In this report, we used the MRL/lpr lupus-prone mouse model, an established model to study NPSLE, to determine the in vivo effects of captopril on Type I IFN and associated immune responses in the periphery and brain and effects on behavior. Administering captopril to MRL/lpr mice decreased expression of IRGs in brain, spleen and kidney, decreased circulating and tissue IFNα levels, decreased microglial activation (IBA-1 expression) and reduced depressive-like behavior. Serotonin levels that are decreased in depression were increased by captopril treatment. Captopril also reduced autoantibody levels in plasma and immune complex deposition in kidney and brain. Thus, ACEi's may have potential for therapeutic use for systemic and NPSLE.
Keywords: angiotensin-converting enzyme inhibitor; captopril; depression; immune complex; lupus-prone mice; neuroinflammation; serotonin; type I interferon.
Copyright © 2020 Nocito, Lubinsky, Hand, Khan, Patel, Seliga, Winfield, Zuluaga-Ramirez, Fernandes, Shi, Unterwald, Persidsky and Sriram.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

