Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs
- PMID: 33042283
- PMCID: PMC7532689
- DOI: 10.7150/thno.47289
Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs
Abstract
Intra- and interindividual variation in drug responses is one major reason for the failure of drug therapy, drug toxicity, and even the death of patients. Precision medicine, or personalized medicine, is a field of medicine that customizes an individual's medical diagnosis and treatment based on his/her genes, microbiomes, environments, etc. Over the past decade, a large number of studies have demonstrated that gut microbiota can modify the efficacy and toxicity of drugs, and the extent of the modification varies greatly from person to person because of the variability of the gut microbiota. Personalized manipulation of gut microbiota is an important approach to rectify the abnormal drug response. In this review, we aim to improve drug efficacy and reduce drug toxicity by combining precision medicine and gut microbiota. After describing the interactions between gut microbiota and xenobiotics, we discuss (1) the effects of gut microbiota on drug efficacy and toxicity and the corresponding mechanisms, (2) the variability of gut microbiota, which leads to variation in drug responses, (3) the biomarkers used for the patient stratification and treatment decisions before the use of drugs, and (4) the methods used for the personalized manipulation of gut microbiota to improve drug outcomes. Overall, we hope to improve the drug response by incorporating the knowledge of gut microbiota into clinical practice.
Keywords: drug efficacy; drug toxicity; gut bacteria engineering; gut microbiota; precision medicine.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


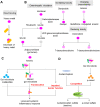

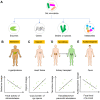


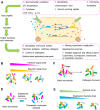
References
-
- Wilkinson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–21. - PubMed
-
- Spear BB, Heath-Chiozzi M, Huff J. Clinical application of pharmacogenetics. Trends Mol Med. 2001;7:201–4. - PubMed
-
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am J Prev Med. 2011;40:585–92. - PubMed
-
- Blieden M, Paramore LC, Shah D, Ben-Joseph R. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev Clin Pharmacol. 2014;7:341–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

