Divergent synthesis of a thiolate-based α-hydroxytropolone library with a dynamic bioactivity profile
- PMID: 33042521
- PMCID: PMC7543996
- DOI: 10.1039/c9ra06383h
Divergent synthesis of a thiolate-based α-hydroxytropolone library with a dynamic bioactivity profile
Abstract
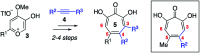
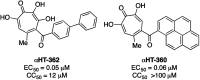
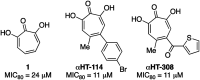
Here we describe a rapid and divergent synthetic route toward structurally novel αHTs functionalized with either one or two thioether or sulfonyl appendages. Evaluation of this library against hepatitis B and herpes simplex virus, as well as the pathogenic fungus Cryptococcus neoformans, and a human hepatoblastoma (HepDES19) revealed complementary biological profiles and new lead compounds with sub-micromolar activity against each pathogen.
Conflict of interest statement
Conflict of Interest MJD, LAM, JET, and RPM are co-inventors on a patent describing αHTs as drug leads for the three diseases described herein.
Figures
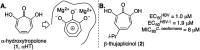


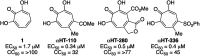
References
-
-
For a few lead examples, see:
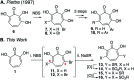
- Piettre S. R. Ganzhorn A. Hoflack J. Islam K. Hornsperger J. M. α-Hydroxytropolones: a new class of potent inhibitors of inositol monophosphatase and other bimetallic enzymes. J. Am. Chem. Soc. 1997;119:3201–3204. doi: 10.1021/ja9634278. - DOI
- Allen N. E. Alborn Jr W. E. Hobbs Jr J. N. A Kirst J. H. 7-Hydroxytropolones: an inhibitor of aminoglycoside 2′′-O-adenyltransferase. Antimicrob. Agents Chemother. 1982;22:824–831. doi: 10.1128/AAC.22.5.824. - DOI - PMC - PubMed
- Budihas S. R. Gorshkova I. Gaidamakov S. Wamiru A. Bona M. K. Parniak M. A. Crouch R. J. McMahon J. B. Beutler J. A. Le Grice S. F. J. Selective inhibition of HIV-1 reverse transcriptase-associated ribonuclease H activity by hydroxylated tropolones. Nucleic Acids Res. 2005;33:1249–1256. doi: 10.1093/nar/gki268. - DOI - PMC - PubMed
-
-
- Banwell M. G. Collis M. P. The palladium-mediated cross-coupling of bromotropolones with organostannanes; application to concise syntheses of β-dolabrin, β-thujaplicin, 7-methoxy-4-isopropyltropolones, and β-thujaplicinol. J. Chem. Soc., Chem. Commun. 1991:1343–1345. doi: 10.1039/C39910001343. - DOI
- Zinser J. Henkel S. Fohlisch B. A novel synthesis of 2-alkoxy-3-hydroxytropones and 2,7-dihydroxytropones from dialkoxy- 8-oxabicyclo[3.2.1]oct-6-en-3-ones. Eur. J. Org. Chem. 2004:1344–1346. doi: 10.1002/ejoc.200300600. - DOI
- Yamatani T. Yasunami M. Takase K. Dehydrotropolones: a benzyne-type Intermediate in the reaction of halotropolones with alkoxides. Tetrahedron Lett. 1970;11:1752–1758. doi: 10.1016/S0040-4039(01)98066-9. - DOI
-
-
For HBV, see:
- Hu Y. Cheng X. Cao F. Huang A. Tavis J. E. β-Thujaplicinol inhibits hepatitis B virus replication by blocking the viral ribonuclease H activity. Antivir. Res. 2013;99:221–229. doi: 10.1016/j.antiviral.2013.06.007. - DOI - PubMed
-
. For HSV-1, see:
- Tavis J. E. Wang H. Tollefson A. E. Ying B. Korom M. Cheng X. Cao F. Davis K. L. Wold W. S. M. Morrison L. A. Inhibitors of nucleotidyltransferase superfamily enzymes suppress herpes simplex virus replication. Antimicrob. Agents Chemother. 2014;58:7451–7461. doi: 10.1128/AAC.03875-14. - DOI - PMC - PubMed
-
. For C. neoformans, see:
- Donlin M. J. Zunica A. Lipnicky A. Garimallaprabhakaran A. K. Berkowitz A. J. Grigoryan A. Meyers M. J. Tavis J. E. Murelli J. R. P. Troponoids can inhibit growth of the human fungal pathogen Cryptococcus neoformans. Antimicrob. Agents Chemother. 2017;61:e02574–16. doi: 10.1128/AAC.02574-16. - DOI - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources

