Adverse Impact of Heavy Metals on Bone Cells and Bone Metabolism Dependently and Independently through Anemia
- PMID: 33042736
- PMCID: PMC7539179
- DOI: 10.1002/advs.202000383
Adverse Impact of Heavy Metals on Bone Cells and Bone Metabolism Dependently and Independently through Anemia
Abstract
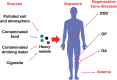
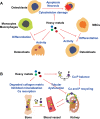
Mounting evidence is revealing that heavy metals can incur disordered bone homeostasis, leading to the development of degenerative bone diseases, including osteoporosis, osteoarthritis, degenerative disk disease, and osteomalacia. Meanwhile, heavy metal-induced anemia has been found to be intertwined with degenerative bone diseases. However, the relationship and interplay among these adverse outcomes remain elusive. Thus, it is of importance to shed light on the modes of action (MOAs) and adverse outcome pathways (AOPs) responsible for degenerative bone diseases and anemia under exposure to heavy metals. In the current Review, the epidemiological and experimental findings are recapitulated to interrogate the contributions of heavy metals to degenerative bone disease development which may be attributable dependently and independently to anemia. A few likely mechanisms are postulated for anemia-independent degenerative bone diseases, including dysregulated osteogenesis and osteoblastogenesis, imbalanced bone formation and resorption, and disturbed homeostasis of essential trace elements. By contrast, remodeled bone microarchitecture, inhibited erythropoietin production, and disordered iron homeostasis are speculated to account for anemia-associated degenerative bone disorders upon heavy metal exposure. Together, this Review aims to elaborate available literature to fill in the knowledge gaps in understanding the detrimental effects of heavy metals on bone cells and bone homeostasis through different perspectives.
Keywords: adverse outcome pathways; anemia; degenerative bone diseases; heavy metals; mode of actions.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Rehman K., Fatima F., Waheed I., Akash M. S. H., J. Cell. Biochem. 2018, 119, 157. - PubMed
-
- Özcan M. M., Aljuhaimi F., Uslu N., Ghafoor K., Mohamed Ahmed I. A., Babiker E. E., Environ. Sci. Pollut. Res. Int. 2019, 26, 28210. - PubMed
-
- Morais S., Costa F., Pereira M., Heavy Met. Hum. Health 2012. 10.5772/29869. - DOI
LinkOut - more resources
Full Text Sources
