Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles
- PMID: 33042751
- PMCID: PMC7539212
- DOI: 10.1002/advs.202001334
Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles
Abstract
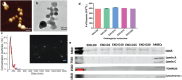
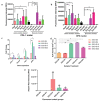
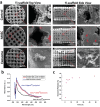
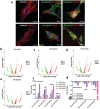
Implantation of stem cells for tissue regeneration faces significant challenges such as immune rejection and teratoma formation. Cell-free tissue regeneration thus has a potential to avoid these problems. Stem cell derived exosomes do not cause immune rejection or generate malignant tumors. Here, exosomes that can induce osteogenic differentiation of human mesenchymal stem cells (hMSCs) are identified and used to decorate 3D-printed titanium alloy scaffolds to achieve cell-free bone regeneration. Specifically, the exosomes secreted by hMSCs osteogenically pre-differentiated for different times are used to induce the osteogenesis of hMSCs. It is discovered that pre-differentiation for 10 and 15 days leads to the production of osteogenic exosomes. The purified exosomes are then loaded into the scaffolds. It is found that the cell-free exosome-coated scaffolds regenerate bone tissue as efficiently as hMSC-seeded exosome-free scaffolds within 12 weeks. RNA-sequencing suggests that the osteogenic exosomes induce the osteogenic differentiation by using their cargos, including upregulated osteogenic miRNAs (Hsa-miR-146a-5p, Hsa-miR-503-5p, Hsa-miR-483-3p, and Hsa-miR-129-5p) or downregulated anti-osteogenic miRNAs (Hsa-miR-32-5p, Hsa-miR-133a-3p, and Hsa-miR-204-5p), to activate the PI3K/Akt and MAPK signaling pathways. Consequently, identification of osteogenic exosomes secreted by pre-differentiated stem cells and the use of them to replace stem cells represent a novel cell-free bone regeneration strategy.
Keywords: bone regeneration; cell‐free scaffolds; exosomes; miRNAs.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Holdsworth D. W., Thornton M. M., Trends Biotechnol. 2002, 20, S34.
-
- Shi Y., Hu G., Su J., Li W., Chen Q., Shou P., Xu C., Chen X., Huang Y., Zhu Z., Cell Res. 2010, 20, 510. - PubMed
LinkOut - more resources
Full Text Sources
