Superior Acetone Selectivity in Gas Mixtures by Catalyst-Filtered Chemoresistive Sensors
- PMID: 33042762
- PMCID: PMC7539217
- DOI: 10.1002/advs.202001503
Superior Acetone Selectivity in Gas Mixtures by Catalyst-Filtered Chemoresistive Sensors
Abstract
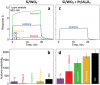
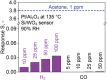
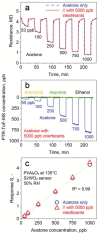
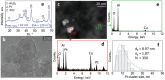
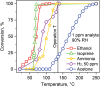
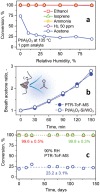
Acetone is a toxic air pollutant and a key breath marker for non-invasively monitoring fat metabolism. Its routine detection in realistic gas mixtures (i.e., human breath and indoor air), however, is challenging, as low-cost acetone sensors suffer from insufficient selectivity. Here, a compact detector for acetone sensing is introduced, having unprecedented selectivity (>250) over the most challenging interferants (e.g., alcohols, aldehydes, aromatics, isoprene, ammonia, H2, and CO). That way, acetone is quantified with fast response (<1 min) down to, at least, 50 parts per billion (ppb) in gas mixtures with such interferants having up to two orders of magnitude higher concentration than acetone at realistic relative humidities (RH = 30-90%). The detector consists of a catalytic packed bed (30 mg) of flame-made Al2O3 nanoparticles (120 m2 g-1) decorated with Pt nanoclusters (average size 9 nm) and a highly sensitive chemo-resistive sensor made by flame aerosol deposition and in situ annealing of nanostructured Si-doped ε-WO3 (Si/WO3). Most importantly, the catalytic packed bed converts interferants continuously enabling highly selective acetone sensing even in the exhaled breath of a volunteer. The detector exhibits stable performance over, at least, 145 days at 90% RH, as validated by mass spectrometry.
Keywords: breath analysis; environmental monitoring; semiconductors; solid‐state gas sensors; wearables.
© 2020 The Authors. Published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Monitoring Lipolysis by Sensing Breath Acetone down to Parts-per-Billion.Small Sci. 2021 Mar 12;1(4):2100004. doi: 10.1002/smsc.202100004. eCollection 2021 Apr. Small Sci. 2021. PMID: 40213359 Free PMC article.
-
Room-Temperature Catalyst Enables Selective Acetone Sensing.Materials (Basel). 2021 Apr 8;14(8):1839. doi: 10.3390/ma14081839. Materials (Basel). 2021. PMID: 33917648 Free PMC article.
-
Catalytic Filter for Continuous and Selective Ethanol Removal Prior to Gas Sensing.ACS Sens. 2020 Apr 24;5(4):1058-1067. doi: 10.1021/acssensors.9b02572. Epub 2020 Mar 26. ACS Sens. 2020. PMID: 32172555
-
Metal oxide-based gas sensors for the detection of exhaled breath markers.Med Devices Sens. 2021 Feb;4(1):e10161. doi: 10.1002/mds3.10161. Epub 2021 Mar 29. Med Devices Sens. 2021. PMID: 33615149 Free PMC article. Review.
-
Nanostructured Metal Oxide-Based Acetone Gas Sensors: A Review.Sensors (Basel). 2020 May 30;20(11):3096. doi: 10.3390/s20113096. Sensors (Basel). 2020. PMID: 32486201 Free PMC article. Review.
Cited by
-
Highly Selective Detection of Benzene and Discrimination of Volatile Aromatic Compounds Using Oxide Chemiresistors with Tunable Rh-TiO2 Catalytic Overlayers.Adv Sci (Weinh). 2021 Feb 1;8(6):2004078. doi: 10.1002/advs.202004078. eCollection 2021 Mar. Adv Sci (Weinh). 2021. PMID: 33747750 Free PMC article.
-
Smart Gas Sensors: Recent Developments and Future Prospective.Nanomicro Lett. 2024 Nov 4;17(1):54. doi: 10.1007/s40820-024-01543-w. Nanomicro Lett. 2024. PMID: 39489808 Free PMC article. Review.
-
Nanowire Array Breath Acetone Sensor for Diabetes Monitoring.Adv Sci (Weinh). 2024 May;11(19):e2309481. doi: 10.1002/advs.202309481. Epub 2024 Mar 13. Adv Sci (Weinh). 2024. PMID: 38477429 Free PMC article.
-
A Strategy to Enhance Humidity Robustness of p-Type CuO Sensors for Breath Acetone Quantification.Small Sci. 2023 Feb 28;3(4):2200096. doi: 10.1002/smsc.202200096. eCollection 2023 Apr. Small Sci. 2023. PMID: 40212766 Free PMC article.
-
Improved isoprene detection performance of Si-doped WO3 films deposited by sputtering and post-annealing.RSC Adv. 2024 Apr 25;14(19):13618-13627. doi: 10.1039/d4ra00184b. eCollection 2024 Apr 22. RSC Adv. 2024. PMID: 38665489 Free PMC article.
References
-
- Acetone Market Size and Forecast, by Application, by Region and Trend Analysis 2014–2024, Hexa Research, Felton, CA: 2017.
-
- Toxicological Review of Acetone, United States Environmental Protection Agency, Washington, DC: 2017.
-
- Kalapos M. P., Biochim. Biophys. Acta 2003, 1621, 122. - PubMed
-
- Güntner A. T., Sievi N. A., Theodore S. J., Gulich T., Kohler M., Pratsinis S. E., Anal. Chem. 2017, 89, 10578. - PubMed
-
- Španěl P., Dryahina K., Rejšková A., Chippendale T. W. E., Smith D., Physiol. Meas. 2011, 32, 22. - PubMed
LinkOut - more resources
Full Text Sources
