A Novel Prognostic Scoring System Integrating Gene Expressions and Clinicopathological Characteristics to Predict Very Early Relapse in Node-Negative Estrogen Receptor-Positive/HER2-Negative Breast Cancer
- PMID: 33042787
- PMCID: PMC7518385
- DOI: 10.3389/fonc.2020.01335
A Novel Prognostic Scoring System Integrating Gene Expressions and Clinicopathological Characteristics to Predict Very Early Relapse in Node-Negative Estrogen Receptor-Positive/HER2-Negative Breast Cancer
Abstract
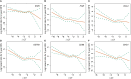
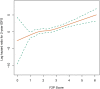
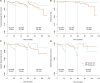
Background: Despite low aggressiveness in tumor biology and high responsiveness to endocrine therapy, subgroups of patients with estrogen receptor-positive/HER2-negative (ER+/HER2-) breast cancer relapse early in the first two years after initiation of endocrine therapy, indicating potential endocrine resistance. Accordingly, we attempted to establish a scoring system to inform the first-2-year prognosis (F2P Score). Methods: Patients with node-negative ER+/HER2- breast cancer and complete data of gene expressions in a 21-gene panel were retrospectively retrieved from Shanghai Jiao Tong University Breast Cancer Database (SJTU-BCDB). The F2P Score was established based on the clinical and genomic variables associated with the first-2-year relapse after shrinkage correction and validated using the bootstrap resampling method. Model performance was quantified by Harrell's concordance-index (C-index) and Bayesian information criteria (BIC). Results: The F2P Score was established by integrating the clinical (age and tumor size) and genomic (ESR1, PGR, BCL2, CD68, GSTM1, and BAG1) variables with a C-index of 0.71 and BIC of 397.46. Bootstrap C-index was 0.72 (95% CI, 0.62-0.81) and BIC was 396.75 (95% CI, 252.37-541.13). A higher score indicated an increased likelihood of a first-2-year relapse, when used as continuous (HR, 2.94; 95% CI, 1.87-4.61) or categorical (HR, 3.68; 95% CI, 1.70-8.00) predictors in multivariate analysis. Both continuous and categorical F2P Score also remained prognostic for overall survival and other endpoints. No significant interaction was observed between the F2P Score and treatment subgroups. Additionally, the F2P Score outperformed the IHC4, clinical treatment score and 21-gene test in predicting first-2-year relapse. Conclusion: The F2P Score reported herein, integrating the clinicopathological and genomic variables, may inform prognosis and endocrine responsiveness. After the benefits and risks have been considered, treatment escalation may be an alternative strategy for patients with a higher score.
Keywords: breast neoplasm; endocrine response; first-2-year relapse; model development; prognosis.
Copyright © 2020 Lin, Wu, Lin, Fei, Chen, Huang, He, Chen, Li, Shen and Zhu.
Figures

Similar articles
-
Real-world data on breast pathologic complete response and disease-free survival after neoadjuvant chemotherapy for hormone receptor-positive, human epidermal growth factor receptor-2-negative breast cancer: a multicenter, retrospective study in China.World J Surg Oncol. 2022 Sep 29;20(1):326. doi: 10.1186/s12957-022-02787-9. World J Surg Oncol. 2022. PMID: 36175898 Free PMC article.
-
Prognostic Value of Modified IHC4 Score in Patients with Estrogen Receptor-Positive Metastatic Breast Cancer.Oncologist. 2020 Aug;25(8):e1170-e1180. doi: 10.1634/theoncologist.2019-1006. Epub 2020 Jun 16. Oncologist. 2020. PMID: 32476192 Free PMC article.
-
Comparison of prognostic and predictive impact of genomic or central grade and immunohistochemical subtypes or IHC4 in HR+/HER2- early breast cancer: WSG-AGO EC-Doc Trial.Ann Oncol. 2016 Jun;27(6):1035-1040. doi: 10.1093/annonc/mdw070. Epub 2016 Feb 18. Ann Oncol. 2016. PMID: 27022068 Clinical Trial.
-
Molecular essence and endocrine responsiveness of estrogen receptor-negative, progesterone receptor-positive, and HER2-negative breast cancer.BMC Med. 2015 Oct 5;13:254. doi: 10.1186/s12916-015-0496-z. BMC Med. 2015. PMID: 26437901 Free PMC article.
-
Clinicopathological factors predicting early and late distant recurrence in estrogen receptor-positive, HER2-negative breast cancer.Breast Cancer. 2016 Nov;23(6):830-843. doi: 10.1007/s12282-015-0649-0. Epub 2015 Oct 14. Breast Cancer. 2016. PMID: 26467036
Cited by
-
Impact of Different Modules of 21-Gene Assay in Early Breast Cancer Patients.Front Endocrinol (Lausanne). 2021 Nov 2;12:759338. doi: 10.3389/fendo.2021.759338. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34795642 Free PMC article.
-
Exploring the Prognostic Value of Tumour-Associated Genes in Clear Cell Renal Cell Carcinoma Through Single-Cell RNA Sequencing Insights.J Cell Mol Med. 2024 Dec;28(24):e70297. doi: 10.1111/jcmm.70297. J Cell Mol Med. 2024. PMID: 39706820 Free PMC article.
-
Prognostic values of clinical and molecular features in HER2 low-breast cancer with hormonal receptor overexpression: features of HER2-low breast cancer.Breast Cancer. 2022 Sep;29(5):844-853. doi: 10.1007/s12282-022-01364-y. Epub 2022 Jun 21. Breast Cancer. 2022. PMID: 35729304 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

