Inflammasome Deletion Promotes Anti-tumor NK Cell Function in an IL-1/IL-18 Independent Way in Murine Invasive Breast Cancer
- PMID: 33042810
- PMCID: PMC7526436
- DOI: 10.3389/fonc.2020.01683
Inflammasome Deletion Promotes Anti-tumor NK Cell Function in an IL-1/IL-18 Independent Way in Murine Invasive Breast Cancer
Abstract
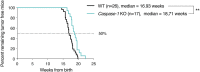
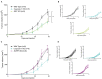
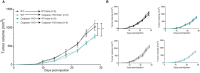
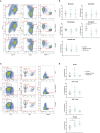
Inflammasomes are molecular complexes that trigger an inflammatory response upon detection of pathogens or danger signals. Recent studies suggest that they are also involved in cancer progression. However, their roles during tumorigenesis remain poorly understood and controversial. Here, we investigated whether inflammasome activation supports mammary tumor growth. Using mouse models of invasive breast cancer, our results demonstrate that the absence of a functional inflammasome impairs tumor growth. Importantly, tumors implanted into inflammasome-deficient mice recruited significantly less neutrophils and more natural killer (NK) cells, and these latter cells displayed a more active phenotype. Interestingly, NK cell depletion abolished the anti-tumoral effect observed in inflammasome-deficient mice, although inflammasome-regulated cytokine neutralization had no effect. Thus, our work identifies a novel role for the inflammasome in supporting mammary tumor growth by attenuating NK cell recruitment and activity. These results suggest that inflammasome inhibition could be a putative target for treating invasive breast cancers.
Keywords: ASC; Caspase-1; NK cells; breast cancer; inflammasome; inflammation.
Copyright © 2020 Guey, Bodnar-Wachtel, Drouillard, Eberhardt, Pratviel, Goutagny, Bendriss-Vermare, Puisieux, Caux, Walzer and Petrilli.
Figures
Similar articles
-
Murine Gammaherpesvirus 68 Pathogenesis Is Independent of Caspase-1 and Caspase-11 in Mice and Impairs Interleukin-1β Production upon Extrinsic Stimulation in Culture.J Virol. 2015 Jul;89(13):6562-74. doi: 10.1128/JVI.00658-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855746 Free PMC article.
-
The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity.Immunity. 2015 Oct 20;43(4):751-63. doi: 10.1016/j.immuni.2015.08.013. Epub 2015 Sep 15. Immunity. 2015. PMID: 26384545
-
Activation of Human NK Cells by Bordetella pertussis Requires Inflammasome Activation in Macrophages.Front Immunol. 2019 Aug 27;10:2030. doi: 10.3389/fimmu.2019.02030. eCollection 2019. Front Immunol. 2019. PMID: 31507615 Free PMC article.
-
NLRP3 Inflammasome: A Possible Link Between Obesity-Associated Low-Grade Chronic Inflammation and Colorectal Cancer Development.Front Immunol. 2018 Dec 11;9:2918. doi: 10.3389/fimmu.2018.02918. eCollection 2018. Front Immunol. 2018. PMID: 30619282 Free PMC article. Review.
-
Inhibiting the inflammasome: one domain at a time.Immunol Rev. 2015 May;265(1):205-16. doi: 10.1111/imr.12290. Immunol Rev. 2015. PMID: 25879295 Free PMC article. Review.
Cited by
-
Prognostic Value of NLRP3 Inflammasome and TLR4 Expression in Breast Cancer Patients.Front Oncol. 2021 Sep 2;11:705331. doi: 10.3389/fonc.2021.705331. eCollection 2021. Front Oncol. 2021. PMID: 34540671 Free PMC article.
-
Downstream Signaling of Inflammasome Pathway Affects Patients' Outcome in the Context of Distinct Molecular Breast Cancer Subtypes.Pharmaceuticals (Basel). 2022 May 24;15(6):651. doi: 10.3390/ph15060651. Pharmaceuticals (Basel). 2022. PMID: 35745570 Free PMC article.
-
Inhibition of NLRP3 inflammasome contributes to paclitaxel efficacy in triple negative breast cancer treatment.Sci Rep. 2024 Oct 21;14(1):24753. doi: 10.1038/s41598-024-75805-3. Sci Rep. 2024. PMID: 39433537 Free PMC article.
-
Harnessing innate immune pathways for therapeutic advancement in cancer.Signal Transduct Target Ther. 2024 Mar 25;9(1):68. doi: 10.1038/s41392-024-01765-9. Signal Transduct Target Ther. 2024. PMID: 38523155 Free PMC article. Review.
-
Inflammasomes in Cancer Progression and Anti-Tumor Immunity.Front Cell Dev Biol. 2022 Apr 20;10:839041. doi: 10.3389/fcell.2022.839041. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35517498 Free PMC article. Review.
References
-
- Kristensen VN, Vaske CJ, Ursini-Siegel J, Van Loo P, Nordgard SH, Sachidanandam R, et al. . Integrated molecular profiles of invasive breast tumors and ductal carcinoma in situ (DCIS) reveal differential vascular and interleukin signaling. Proc Natl Acad Sci USA. (2012) 109:2802–7. 10.1073/pnas.1108781108 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

