Human Cytomegalovirus Inhibits Autophagy of Renal Tubular Epithelial Cells and Promotes Cellular Enlargement
- PMID: 33042861
- PMCID: PMC7522221
- DOI: 10.3389/fcimb.2020.00474
Human Cytomegalovirus Inhibits Autophagy of Renal Tubular Epithelial Cells and Promotes Cellular Enlargement
Abstract
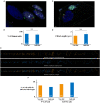
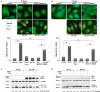
Human Cytomegalovirus (HCMV) is a frequent opportunistic pathogen in immunosuppressed patients, which can be involved in kidney allograft dysfunction and rejection. In order to study the pathophysiology of HCMV renal diseases, we concentrated on the impact of HCMV infection on human renal tubular epithelial HK-2 cells. Our aim was to develop a model of infection of HK-2 cells by using the viral strain TB40/E, that contains the extended cell tropism of clinical isolates and the efficient viral multiplication in cell culture of laboratory-adapted strains. We observed that HK-2 cells can be infected by HCMV and expressed viral antigens, but they do not produce extracellular viral particles. We then studied the interplay of HCMV with ciliogenesis and autophagy. Primary cilium (PC) is a stress sensor important to maintain renal tissue homeostasis that projects from the apical side into the lumen of tubule cells. PC formation and length were not modified by HCMV infection. Autophagy, another stress response process critically required for normal kidney functions, was inhibited by HCMV in HK-2 cells with a reduction in the autophagic flux. HCMV classically induces an enlargement of infected cells in vivo and in vitro, and we observed that HCMV infection led to an enlargement of the HK-2 cell volume. Our results constitute therefore an excellent starting point to further explore the role of these mechanisms in renal cells dysfunction.
Keywords: autophagy; cellular size; cytomegaly; cytopathic effect; human cytomegalovirus; polarization; primary cilium; renal cells.
Copyright © 2020 López Giuliani, Hernández, Tohmé, Taisne, Roldán, García Samartino, Lussignol, Codogno, Colombo, Esclatine and Delgui.
Figures